Translate this page into:
Direct immunofluorescence demystified: Essential insights and recent advances for dermatologists
Corresponding author: Dr. Debajyoti Chatterjee, Department of Histopathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. devchat1984@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Phiske MM, Khullar G, Padhiyar JK, Hosthota A, Chatterjee D. Direct immunofluorescence demystified: Essential insights and recent advances for dermatologists. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_95_2024
Abstract
Direct immunofluorescence (DIF) is widely used in dermatopathology for the diagnosis of autoimmune blistering diseases (AIBDs), cutaneous vasculitis, and connective tissue disorders. Although it is easy and useful to perform, it needs technical expertise and experience for proper interpretation. The yield of DIF depends on multiple factors including the adequacy, transportation, storage, processing, and interpretation of the biopsy specimen. Effective collaboration between the dermatologist and dermatopathologist along with meticulous clinico-pathological correlation is crucial for accurately interpreting DIF in the appropriate clinical context.
In this narrative review of DIF in dermatology, we discuss the indications of DIF, recent updates on the selection of optimum biopsy sites, basic techniques of DIF including the classical transport medium and its alternatives, processing and staining technique, patterns in various diseases, advancements such as serration pattern analysis, and latest recommendations on the use of DIF in cutaneous disorders.
Keywords
Direct immunofluorescence
autoimmune blistering diseases
cutaneous vasculitis
serration pattern
Introduction
The American pathologist Albert Coons first employed immunofluorescence (IF) to visualise pneumococcal antigens in 1941.1 The use of DIF in various dermatologic diseases including lupus erythematosus was first reported by Burnham et al in 1963.2 In 1967, Jordan’s group presented a groundbreaking study demonstrating the detection of basement membrane zone (BMZ) antibodies using DIF and IIF in bullous pemphigoid (BP).3 IF has since revolutionised the classification of autoimmune bullous disorders and has proven invaluable for diagnosing and prognosticating disorders such as cutaneous connective tissue disorders, genetic blistering diseases, and vasculitis.
IF is now an essential diagnostic tool in dermatology. Based on the technique used, IF is classified as:
-
(1)
Direct immunofluorescence (DIF)
-
(2)
Indirect-immunofluorescence (IIF)
-
(3)
Indirect immunofluorescence complement-fixation (IIF-CF)
-
(4)
Immuno-electron microscopy and
-
(5)
In this review of DIF in dermatology, we cover its indications, recent updates on biopsy site selection, basic techniques (including classical transport medium and alternatives), processing and staining techniques, disease patterns, advancements like serration pattern analysis, and the latest recommendations for DIF usage in cutaneous disorders.
Principles of DIF
DIF uses fluorophore or fluorochrome labelled antibodies which interact with a patient’s tissue when incubated to form antigen-antibody conjugates. These can then be visualised under an ultraviolet microscope.7,8 Ideal fluorophores possess high retention and stability, establishing covalent bonds with proteins and emitting fluorescence within the visible light spectrum.9 The most frequently utilised fluorophores include fluorescein isothiocyanate (FITC), generating an apple green hue, and tetramethyl-rhodamine isothiocyanate (TRITC), which emits a red fluorescence.10 Antibodies to IgG, IgG4, IgM, IgA subclasses and sometimes to fibrinogen and C3 (complement 3) are used in DIF.
Indications for DIF
DIF serves as the gold standard for diagnosing and monitoring disease activity in autoimmune bullous disorders (AIBDs). As DIF findings may be similar in many sub-epidermal AIBDs, additional diagnostic techniques such as serration pattern analysis, IIF on salt-split skin, enzyme-linked immunosorbent assay (ELISA), and immunoblotting are often employed to differentiate these conditions.
DIF is also useful as a supportive investigation in connective tissue disorders such as SLE, and cutaneous vasculitis (especially IgA vasculitis).11,12 Immunofluorescence mapping (a modification of DIF), is used for the diagnosis of epidermolysis bullosa.
Site of Biopsy and transport for DIF
The selection of an appropriate biopsy site is critical for obtaining a representative specimen. Table 1 outlines recommended biopsy sites for various disorders.
| Disorder | Site of biopsy | Type of biopsy |
|---|---|---|
| Autoimmune bullous disease. |
Perilesional skin (within 1 cm of bulla). Avoid: 1. Bullous lesions (immune reactants consumed here due to ongoing inflammation so DIF may be falsely negative 2. Biopsy from uninvolved skin distant from bulla and from lower extremity (in case of BP) may give false negative results.13 |
Punch biopsy of 3–4 mm |
| Dermatitis herpetiformis | Perilesional skin (within 1 cm of bulla). | Broad, deep shave biopsy (instead of punch biopsy) |
| Vasculitis | Recent purpuric lesion (< 24 hours old) located most proximally | Punch biopsy of 3–4 mm |
| DLE, lichen planus and porphyria cutanea tarda | Lesional biopsy | Punch biopsy of 3–4 mm |
| SLE | Skin from both lesional and non-lesional areas, including from sun-exposed (e.g., shoulder) and non-sun-exposed sites (e.g., volar forearm/buttock)8,12,13 | Punch biopsy of 3–4 mm |
DLE: Discoid lupus erythematosus, SLE: systemic lupus erythematosus
Formalin, commonly used in histopathology for tissue fixation, can degrade antigens in specimens intended for DIF analysis. Therefore, the biopsy sample for DIF should be taken prior to obtaining samples for routine histopathology in order to prevent contamination of forceps and DIF specimens with formalin. In case of accidental contamination, the specimen should be removed immediately from formalin and rinsed with normal saline, or better still, a fresh biopsy specimen should be obtained.13
Oral cavity as a site of biopsy
Oral biopsies for DIF are technically difficult to perform due to poor accessibility, the need for minimal tissue handling, and the innate fragility of the mucosa. A recent study has shown that a routine buccal punch biopsy of the uninvolved mucosa is as sensitive as a perilesional biopsy (within 1 cm of lesion) in the diagnosis of oral pemphigus vulgaris and multi-site mucous membrane pemphigoid.14
The intact epithelium is easier to obtain from the buccal mucosa as compared to the thin and friable gingiva. Gingival biopsies often result in a falsely negative DIF due to chronic inflammation around the gingiva. Additionally, they may cause a permanent periodontal defect leading to plaque accumulation.14
In pure gingival mucous membrane pemphigoid, a biopsy from the reflected alveolar mucosa adjacent to the area of gingival inflammation is preferred. Obtaining gingival specimens with an intact epithelium is critical for histological and DIF evaluation. In addition to the standard perilesional 3-mm punch biopsy, a “stab-and-roll” technique and a “peeling” technique have also been used to obtain a mucosal biopsy.11
Other substrates for DIF
-
1.
Hair
The outer root sheath of the hair follicle is structurally analogous to the epidermis. The diameter of scalp terminal hair is almost twice that of vellus hair at other body sites. Thus, the total volume of desmosomal structures is greater per unit area of the scalp than at any other epidermal site. Pemphigus antigens are found throughout the outer root sheath of the hair follicle and in the dermal bulb matrix.
Wilson et al. first described the intercellular deposition of immunoreactants in the outer root sheath of the terminal hair follicle in longitudinal sections of follicular-oriented scalp biopsies.15 Subsequently, Tanasilovic et al. showed the utility of DIF of plucked hair in the diagnosis of pemphigus and observed that the sensitivity and specificity of DIF of plucked hair are comparable with that of anti-desmoglein 1 and 3 antibodies as detected by ELISA.16
Five or more anagen hairs, plucked with forceps, are collected. The DIF of both fresh and frozen hair samples (stored for two weeks at –20°C) showed positive results in all patients with active PV and PF. Mounting entire hairs, rather than sections is easier. The sensitivity of DIF of plucked hair is 80–100% and positive results have been documented in cases with only mucosal involvement. 17,18
When monitoring immunological remission in pemphigus, plucked hair provides a simpler, more specific, sensitive, and less invasive alternative to skin biopsy regardless of scalp involvement, thereby avoiding repeated skin biopsies.17,18 However, inadequate samples or lack of expertise in processing hair specimens may result in negative outcomes.
-
2.
Formalin fixed paraffin-embedded (FFPE) tissue
If only formalin-fixed paraffin-embedded (FFPE) tissue is available (e.g., immunobullous or connective tissue disease is not initially suspected, or if frozen tissue is unavailable) DIF can be performed after digestion with proteinase or protease K. However, DIF performed on FFPE samples may show decreased staining and sensitivity and the presence of artefactual background staining of IgG in the epidermis could potentially result in a misdiagnosis of pemphigus.
This technique shows best results in diagnosing bullous pemphigoid and pemphigoid gestationis (with a sensitivity of 79%).19 The diagnosis of IgA vasculitis and dermatitis herpetiformis can pose challenges due to the difficulty in demonstrating IgA deposits.
-
3.
Tzanck smears
DIF may also be performed on Tzanck smears particularly in settings where skin biopsy facilities are not available or lesions are located on mucosal sites that are not easily accessible for biopsy. It provides a straightforward, quick, and non-invasive option compared to skin or mucosal biopsy, with sensitivity ranging from 40% to 87.8%.20,21 The antigenicity in the air-dried smears kept at room temperature is retained for up to 10 days. Positive smears show bright green fluorescence at the margins of the acantholytic cells or in the intercellular spaces if clumps of acantholytic cells are present. However, non-specific background staining in smears prepared from inflammatory lesions is an important limitation.
Handling, Transportation, Processing and Storage of Specimens for DIF
Transportation
-
1.
Michel’s medium (MM) Michel’s medium is a pH-neutral buffered solution containing ammonium sulfate, N-ethylmaleimide, potassium citrate buffer, magnesium sulfate, and distilled water. It is commonly utilised to transport specimens for DIF testing. Unused media should be stored in a refrigerator (2–8°C). If there is ammonium sulfate precipitation, crystals should be dissolved at room temperature before use. The expiration date of the media ranges from 12 to 18 months. Specimens preserved in Michel’s solution can be stored at room temperature for at least 2 weeks and potentially for 1 to 6 months without compromising signal integrity.11
-
2.
Saline:
Phosphate buffered saline (PBS) or normal saline is routinely used to transport biopsy samples that can be transported to the laboratory within a few hours after taking the biopsy. Normal saline has shown superior results in DIF than in MM.22
Advantages of saline as a transport medium include its inexpensive nature and ready availability in all clinics. It significantly reduces background fluorescence in the dermis, particularly with IgG, and may enhance the cutting properties of skin biopsies. Also, saline could potentially induce an artificial split at the dermo-epidermal junction (DEJ) facilitating the detection of immunoreactants at the basement membrane zone (BMZ) and subepidermal blood vessel walls.
Disadvantages include the need to be transported to the laboratory within 24 hours, as immunoreactants undergo rapid degradation in saline after 48 hours especially in tropical and hot climates. Additionally, it is not a suitable medium for antigen mapping in genetic disorders because it may induce an artificial split at the DEJ.4,11
-
3.
Honey:
Honey has recently been shown to serve as an effective transport medium for DIF specimens in resource-poor settings. It is believed to preserve cells by preventing autolysis and putrefaction. The optimum preservation time for samples is approximately two weeks; beyond this period, there is a deterioration of immunoreactants in the biopsy sample.23
Tissue processing and staining
Processing and staining of skin biopsy specimens for DIF has been detailed in Figure 1.
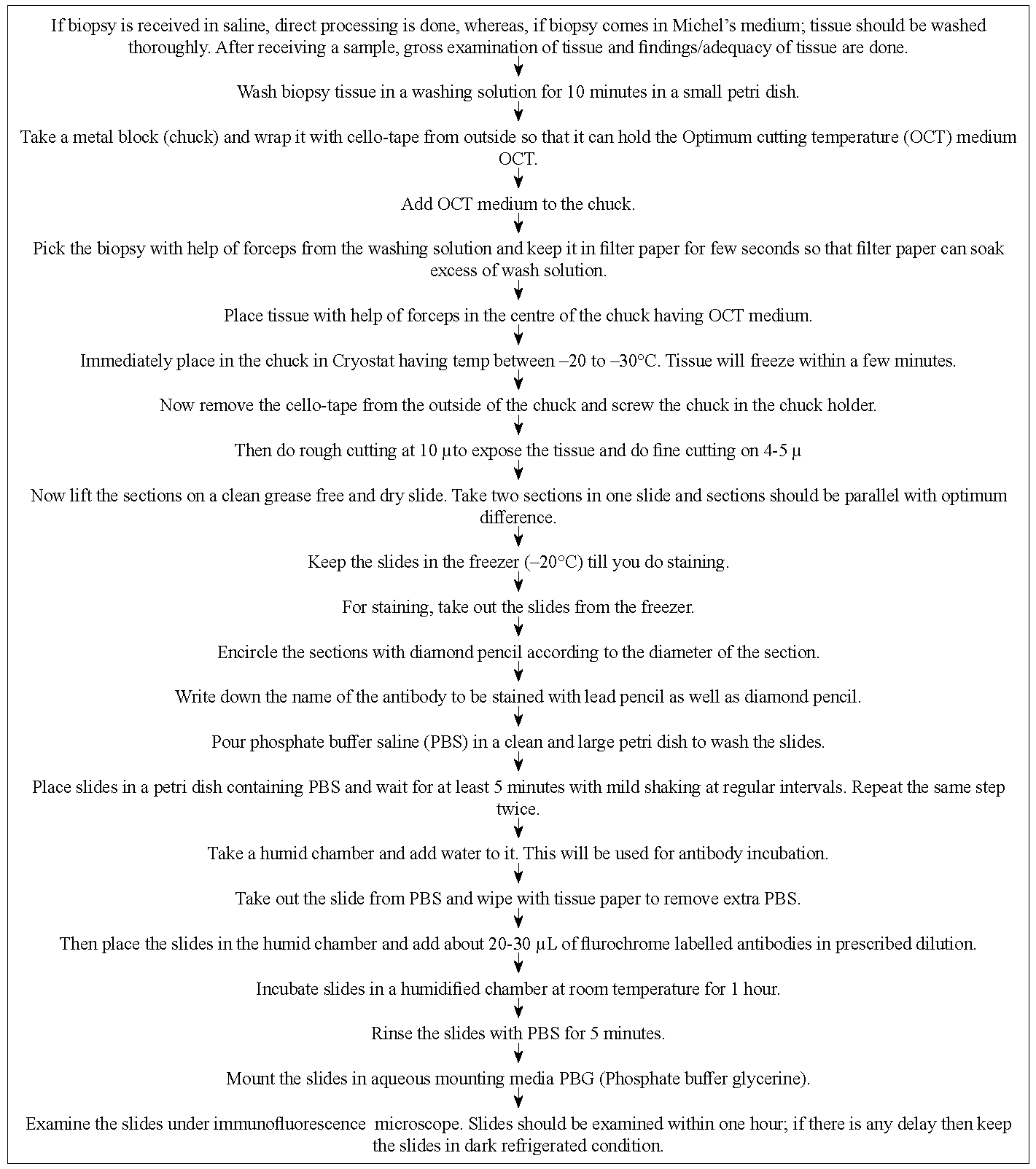
- Processing and staining skin biopsy for DIF.
Other special techniques:
-
1.
Direct-salt split technique:
Immunofluorescence of salt-split skin serves as an effective tool for differentiating various subepidermal autoimmune blistering diseases. Immune binding on either the epidermal (roof) or dermal (floor) side provides crucial diagnostic insights. Both direct and indirect IF can be performed on salt-split skin, with the latter being more commonly used. In the indirect method, patient serum is incubated with control salt-split skin.
However, in the direct salt-split technique the patient’s skin biopsy is incubated in 10–15 mL of 1 mol/L sodium chloride at 40°C for 72 hours, after which the epidermis is separated using fine forceps.24
-
2.
DIF of hair:
Details of this technique have been outlined earlier in the section on “Hair” under “Other substrates for DIF”
Slide storage
After staining, the slides should be stored between 2–8°C in a refrigerator and should be kept in the dark. As the fluorescence fades in 10–14 days, the slides should be interpreted soon after staining. However, using permanent mounting media and antifade reagents, the DIF slides can be preserved for up to 30 days at 2–8°C.25,26
Interpretation of DIF
Slides are evaluated qualitatively and semi-quantitatively on a 0–3+ or 0–4+ scale, as per the convenience of the pathologist. A semi-quantitative assessment of intensity of staining is given as none (0), trace-mild (1+), moderate (2+), and strong (3+/4+). Photographic records are obtained for pertinent positive staining and case-specific positive staining.
DIF is interpreted based on the following points:
-
1.
Type and intensity of immune deposits
-
2.
Pattern of immune deposits (linear/granular/ band like)
-
3.
Location of immune deposits (epidermis, BMZ, dermis, blood vessels, adnexal structures, etc.)
-
4.
Clinical and histological correlation.
The DIF patterns in different conditions have been depicted in Table 2 and Figures 2–13.
| Disease entity | Antibody | Pattern of staining |
|---|---|---|
| Pemphigus vulgaris/ foliaceous/ vegetans | IgG and C3 |
Skin: Linear deposition along intercellular junction in the epidermis and adnexal structures (fishnet pattern)40 Plucked anagen hair: fishnet pattern of immune deposits along the outer root sheath or hair bulb |
| IgA pemphigus | IgA and C3 | Linear deposition along intercellular junction in the epidermis and adnexal structures (fishnet pattern) |
| Paraneoplastic pemphigus | IgG and C3 | Linear deposition along intercellular junction in the epidermis and adnexal structures (fishnet pattern) and/ or linear deposition along epidermal basement membrane zone [Figure 6]41 |
| Pemphigus erythematosus | IgG and C3 | Linear deposition along intercellular junction in the epidermis and adnexal structures (fishnet pattern) and granular deposition along epidermal basement membrane zone |
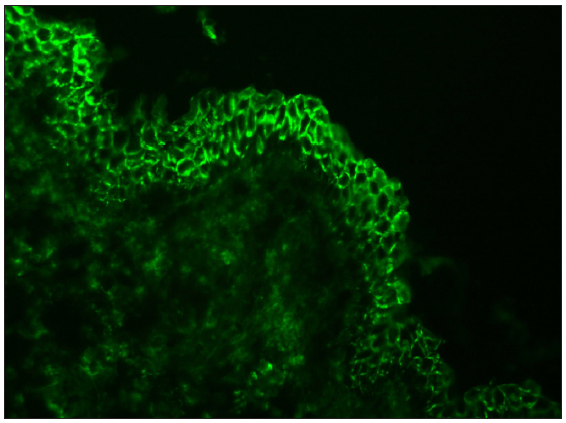
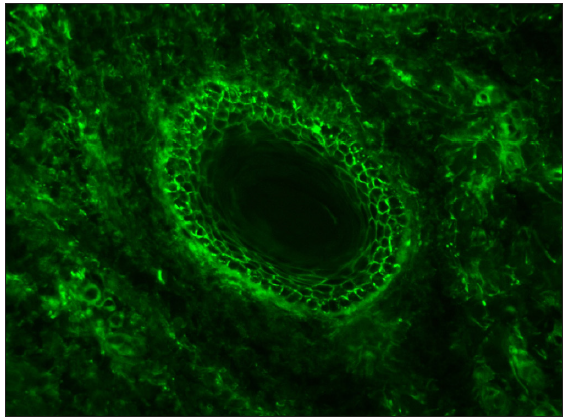
| Bullous pemphigoid/mucous membrane pemphigoid | IgG and C3 | Linear deposition along epidermal/epithelial basement membrane zone in ‘n’ serration pattern [Figures 7–8]35,36,37,42,43 |
| Epidermolysis bullosa acquisita | IgG and C3 (+ IgA) | Linear deposition along epidermal/ epithelial basement membrane zone in ‘u’ serration pattern |
| Dermatitis herpetiformis | IgA + C3 |
Granular deposition along tip of papillary dermis [Figure 9]44-46 Also, linear streaks (ie, fibrillar pattern) and IgA deposition within vessel walls, around hair follicles and along the BMZ of adnexal structures11 |
| Linear IgA disease/ chronic bullous dermatosis of childhood | IgA + C3 | Linear deposition along epidermal basement membrane zone [Figure 10] |
| Discoid lupus erythematosus/ systemic lupus erythematosus | IgG, IgA, IgM, C3, C1q (in various combination) | Granular deposit along epidermal basement membrane zone, adnexal structures, and dermal blood vessels [Figures 11 and 12] |
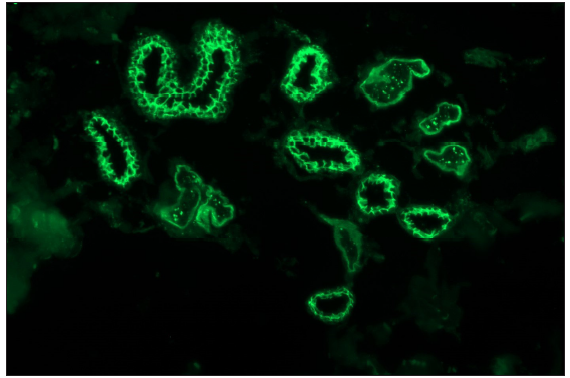
| IgA vasculitis | IgA + C3 | Granular deposit along wall of the dermal capillaries [Figure 13]29,30 |
| Porphyria and pseudoporphyria | IgG, IgM, C3, membrane attack complex | Homogeneous deposition along epidermal basement membrane zone and dermal blood vessels |
- Pemphigus vulgaris showing linear IgG deposition in the epidermis along an intercellular junction giving ‘fishnet’ appearance (fluorescein isothiocyanate, 200x).
- Pemphigus vulgaris showing linear IgG deposition in the hair follicle along an intercellular junction giving ‘fishnet’ appearance (fluorescein isothiocyanate, 200x).
- Pemphigus vulgaris showing linear IgG deposition in the eccrine ducts along an intercellular junction (fluorescein isothiocyanate, 200x).
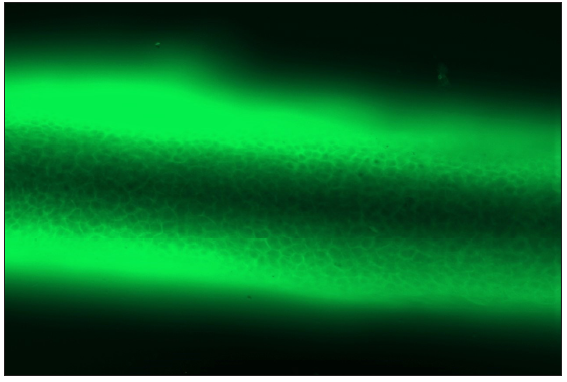
- DIF on plucked anagen hair of pemphigus vulgaris patient showing linear IgG deposition along intercellular junction in the outer root sheath (fluorescein isothiocyanate, 200x).
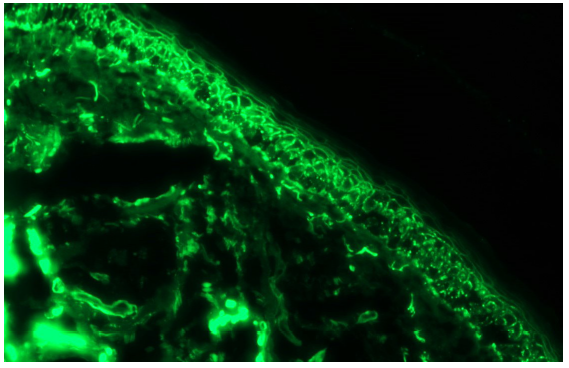
- Paraneoplastic pemphigus showing linear IgG deposition in the epidermis along intercellular junction and linear deposition along dermo-epidermal junction (fluorescein isothiocyanate, 200x).
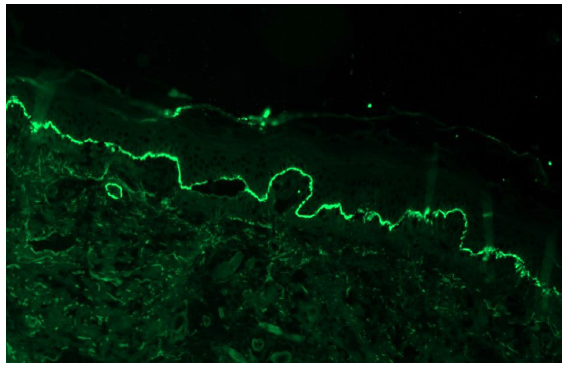
- Bullous pemphigoid showing linear IgG deposition along dermo-epidermal junction (fluorescein isothiocyanate, 100x).
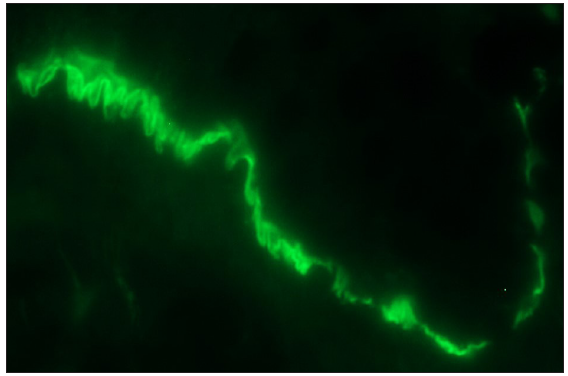
- Bullous pemphigoid showing ‘n’ serration pattern of IgG deposition along dermo-epidermal junction (fluorescein isothiocyanate, oil immersion, 1000x).
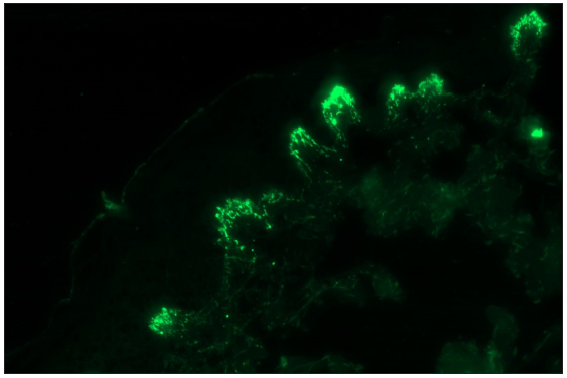
- Granular IgA deposition along the tip of the papillary dermis in a case of dermatitis herpetiformis (fluorescein isothiocyanate, 400x).
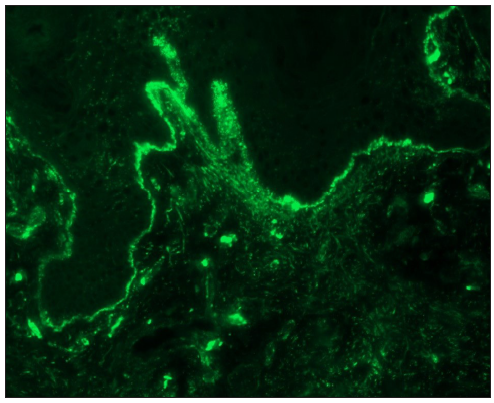
- Linear IgA disease showing linear IgA deposition along dermo-epidermal junction (fluorescein isothiocyanate, 100x).
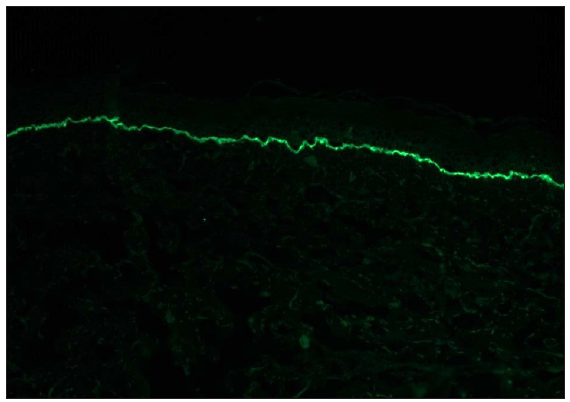
- A case of discoid lupus erythematosus showing granular IgG deposition along dermo-epidermal junction and dermal capillaries (fluorescein isothiocyanate, 200x).
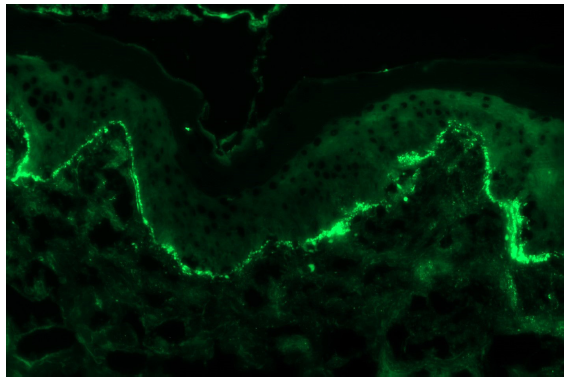
- A case of discoid lupus erythematosus showing granular IgA deposition along dermo-epidermal junction (fluorescein isothiocyanate, 200x).
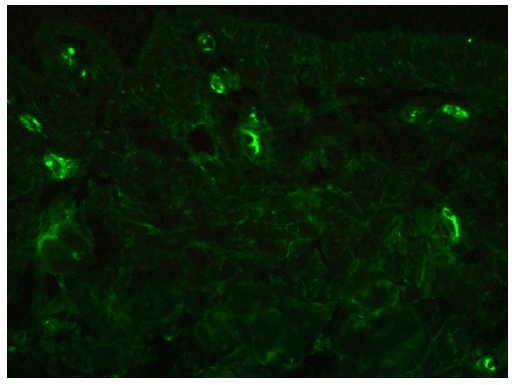
- A case of IgA vasculitis showing granular IgA deposition along upper dermal capillaries (fluorescein isothiocyanate, 400x).
Figure 14 illustrates the approach to subepidermal AIBDs based on DIF and salt split IIF findings.
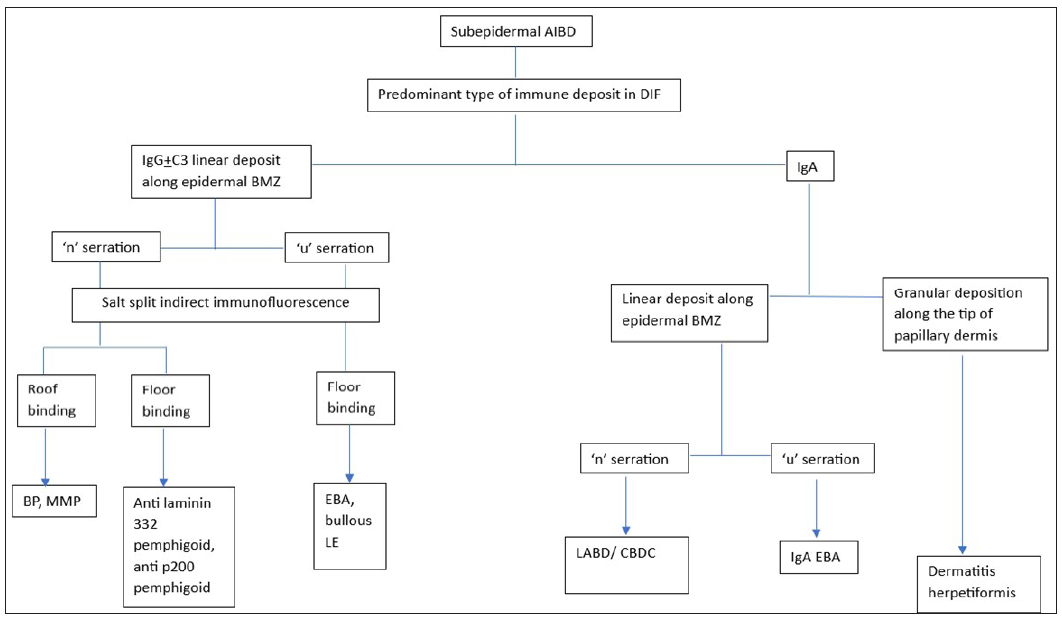
- Flowchart showing diagnostic approach to subepidermal autoimmune blistering disorders based on direct immunofluorescence and salt split indirect immunofluorescence. (AIBD: Autoimmune blistering disorder, BP: Bullous pemphigoid, MMP: mucous membrane pemphigoid, EBA: epidermolysis bullosa acquisita, LE: lupus erythematosus, LABD: linear IgA bullous disorder, CBDC: chronic blistering disorder of childhood, BMZ: basement membrane zone, DIF: direct immunofluorescence).
Reliability of DIF
Sensitivity of DIF and causes of false negative results
In general, DIF is highly sensitive for autoimmune bullous diseases (AIBDs) and cutaneous vasculitis (such as IgA vasculitis) (>90%). DIF is highly sensitive but has limited specificity in the pemphigus group of diseases, while it is very sensitive and specific (90–98%) in the pemphigoid group of disorders.11 However, the sensitivity of DIF is low (27–41%) for the diagnosis of paraneoplastic pemphigus.
The lupus band test has variable sensitivity and specificity in cutaneous lupus erythematosus. It is more sensitive (80–93%) in SLE as compared to DLE (40–60%), however, it has high specificity (80–90%) for acute cutaneous LE.27,28 The sensitivity and specificity of DIF is almost similar for non-lesional sun protected as well as sun exposed areas in SLE.28
False negative results in DIF can result from:
Preanalytical Factors
-
1.
Contamination with formalin
-
2.
Delay in transporting biopsies, especially when sent in saline
-
3.
Use of inadequate transport medium
-
4.
-
5.
Low antibody titres or prior treatment
-
6.
Absence of epidermis or epithelium (common in biopsies taken from the buccal mucosa in autoimmune blistering diseases (AIBDs)
-
7.
Lesional specimens in AIBDs where immunoreactants have already been consumed
Analytical Factors:
-
1.
Incubation not done in moist chamber
-
2.
Inadequate washing of sample (especially when it is transported using Michel’s medium)
Advantages and disadvantages of DIF
The advantages of DIF include:
-
1.
Enhanced intensity of illumination
-
2.
Clearer and brighter image resolution
-
3.
Easy to perform
-
4.
Flexibility to utilise different antibodies for fluorescence
-
5.
Signal amplification capabilities
-
6.
Specific targeting ability
-
7.
Diagnostic and analytical capacity (when clinical features or histopathology are inconclusive, a diagnosis can be solely based on DIF findings. The demonstration of immune complexes in skin biopsies at various locations such as intraepidermal, dermoepidermal junction (DEJ), and dermal blood vessels aids in diagnosis)31
-
8.
Discrimination between immune-mediated bullous lesions and others
-
9.
Definitive diagnosis in certain diseases, such as the fish-net pattern of pemphigus and IgA positivity in linear IgA dermatosis11
-
10.
Facilitates monitoring of response to therapy and can predict relapse in patients in remission (in pemphigus, early positivity in DIF and increased complement deposition indicate relapse in patients)
-
11.
Disadvantages of DIF:
-
1.
Expensive
-
2.
Photobleaching: Fading occurs under intense illumination
-
3.
Formation of protein cross-links in fixed tissue samples
-
4.
Loss of tissue morphology and relevant antigens
-
5.
Requires strict processing and optimal antibody usage determination
-
6.
Immunoreactivity restoration may be necessary if not stored in darkness 33
Advances in DIF
-
1.
Newly recognized DIF patterns in pemphigus In recent studies examining DIF patterns in pemphigus, researchers have observed deposits of IgG4 using a single-step approach. These deposits appeared as small dots under 400x to 600x magnification, resembling ‘dew drops on a spider web,’ contrasting with the textbook ‘fishnet or chicken wire’ pattern of IgG deposition. They suggest that the spotted pattern seen in both extrafollicular and follicular epithelium could be a distinctive sign of pemphigus, given its regular appearance, and is unlikely to be caused by procedural errors or unrelated phenomena.34
-
2.
Serration pattern analysisSerration pattern analysis is an easy, inexpensive, and accurate method for classifying subepidermal AIBDs that provides important diagnostic clues for subepidermal AIBDs.
The linear IgG deposition along the dermo-epidermal junction can be categorised into ‘u’ and ‘n’ serration patterns when observed under high magnification (600x to 1000x). Conditions such as EBA and bullous lupus erythematosus typically exhibit a ‘u’ serration pattern, while most pemphigoid disorders (bullous pemphigoid, mucous membrane pemphigoid, anti-p-200 pemphigoid, and anti-laminin-332 pemphigoid) display an ‘n’ serration pattern.35-37
Recommendations for DIF
Reimann et al.38 in 2021 published recommendations for the evaluation of DIF testing and evaluation as:
-
1.
Limit the panel to IgG, IgA, and C3 in suspected immunobullous disease, and to IgA, C3, and fibrin in suspected vasculitis [Table 3]. The standard practice of reflexive antibody testing using a 6-antibody panel for all DIF biopsies is unnecessary. A DIF protocol tailored to the submitting diagnosis may enhance cost-effectiveness without compromising test sensitivity and specificity.
-
2.
Where the clinical impression is broad or nonspecific (i.e., immunobullous disease), the addition of IgM and fibrin to the basic IgG, IgA, and C3 panel may be necessary.
-
3.
Technicians should be trained to use DIF protocols based on the submitting diagnosis, including lists of specific clinical differential diagnoses for which a 3-antibody panel rather than a full panel is warranted.
-
4.
DIF using non-lesional buccal mucosa was found to be superior to histological and serological tests for diagnosing mucous membrane pemphigoid. The procedure is technically easy and has high diagnostic value.39
-
5.
Consider adopting a more cost-conscious and high-value approach to utilising DIF for clinical purposes, which both local and national educational societies should be incorporated into their curriculum planning.
-
6.
Due to the observed lack of utility in diagnosing suspected porphyria, lichen planus (LP), or lupus erythematosus, DIF is not recommended for these conditions.
-
7.
Accurate communication between dermatologists and dermatopathologists are essential.
| Clinical possibility | Biopsy for DIF | Panel of immunoreactants |
|---|---|---|
| Immunobullous disorder | Recommended | IgG, IgA, C3 |
| Vasculitis | Recommended, primarily for suspected Henoch-Schönlein purpura | IgA, C3, fibrin |
| Connective tissue disease | Not routinely recommended | IgA, C3, IgM, IgG |
| Lichen planus | Not routinely recommended | - |
| Lichen planus pemphigoides | Recommended | IgG, IgA, C3 |
| Porphyria | Not routinely recommended | - |
Conclusion
Although DIF is an extremely useful diagnostic tool, it should always be used in conjunction with histopathology and clinical features for best results. DIF microscopy not only serves to confirm the diagnosis in AIBDs but also directs further immunological tests such as enzyme-linked immunosorbent assay (ELISA) and Western blotting. Although DIF can be challenging to perform, clinicians can maximise the yield by carefully selecting the site, employing proper technique, ensuring appropriate tissue handling, and coordinating effectively with dermatopathologists. Importantly, accurate interpretation of the results requires incorporating clinical and histological findings along with good clinico-pathological correlation.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol Med. 1941;47:200-2.
- [Google Scholar]
- The application of the fluorescent antibody technic to the investigation of lupus erythematosus and various dermatoses. J Invest Dermatol. 1963;41:451-6.
- [CrossRef] [PubMed] [Google Scholar]
- Techniques of immunofluorescence and their significance. Indian J Dermatol Venereol Leprol. 2008;74:415-9.
- [CrossRef] [PubMed] [Google Scholar]
- The immunofluorescence techniques in the diagnosis of endocrine autoimmune diseases. Auto Immun Highlights. 2012;3:67-78.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Immunofluorescence in dermatology. Indian J Dermatol Venereol Leprol. 2012;78:677-91.
- [CrossRef] [PubMed] [Google Scholar]
- A Starter Guide to Immunofluorescence Testing in Dermatology. Cutis. 2021;108:E23-E26.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of immunofluorescence in dermatology. Indian Dermatol Online J. 2017;8:1-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Direct and indirect immunofluorescence. An Bras Dermatol. 2010;85:490-500.
- [CrossRef] [PubMed] [Google Scholar]
- Immunofluorescence in oral mucosal diseases – A review. Oral Surg Oral Med Oral Radiol. 2014;2:6-10.
- [Google Scholar]
- Practical direct immunofluorescence. Am J Dermatopathol. 2020;42:75-85.
- [CrossRef] [PubMed] [Google Scholar]
- Direct immunofluorescence testing in the diagnosis of immunobullous disease, collagen vascular disease, and vascular injury syndromes. Dermatol Clin. 2012;30:763-98.
- [CrossRef] [PubMed] [Google Scholar]
- Skin biopsy: Biopsy issues in specific diseases. J Am Acad Dermatol. 2016;74:1-16.
- [CrossRef] [PubMed] [Google Scholar]
- The optimal oral biopsy site for diagnosis of mucous membrane pemphigoid and pemphigus vulgaris. Br J Dermatol. 2020;182:747-53.
- [CrossRef] [PubMed] [Google Scholar]
- Pemphigus and the terminal hair follicle. J Cutan Pathol. 1991;18:428-31.
- [CrossRef] [PubMed] [Google Scholar]
- Direct immunofluorescence of the outer root sheath in anagen and telogen hair in pemphigus vulgaris and pemphigus foliaceus. Australas J Dermatol. 2014;55:e74-6.
- [CrossRef] [PubMed] [Google Scholar]
- Demonstration of pemphigus-specific immunofluorescence pattern by direct immunofluorescence of plucked hair. Int J Dermatol. 2009;48:1187-9.
- [CrossRef] [PubMed] [Google Scholar]
- Immunofluorescence of the outer root sheath: An aid to diagnosis in pemphigus. Clin Exp Dermatol. 2011;36:298-301.
- [CrossRef] [PubMed] [Google Scholar]
- The value of direct immunofluorescence on proteinase-digested formalin-fixed paraffin embedded skin biopsies. Am J Dermatopathol. 2018;40:111-7.
- [CrossRef] [PubMed] [Google Scholar]
- Role of direct immunofluorescence on Tzanck smears in pemphigus vulgaris. Diagn Cytopathol. 2007;35:403-7.
- [CrossRef] [PubMed] [Google Scholar]
- Direct immunofluorescence analysis of oral tzanck smears for pemphigus vulgaris: A diagnostic test. J Oral Pathol Med. 2021;50:1050-6.
- [CrossRef] [PubMed] [Google Scholar]
- Enhanced diagnostic immunofluorescence using biopsies transported in saline. BMC Dermatol. 2004;4:10.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A comparative study of Michel’s medium versus honey as a transport medium for skin specimens prior to direct immunofluorescence microscopy and antigen mapping. J Cutan Pathol. 2019;46:729-35.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of salt split technique of immunofluorescence in bullous pemphigoid. Indian J Dermatol Venereol Leprol. 2002;68:330-3.
- [PubMed] [Google Scholar]
- Durability of direct immunofluorescence (DIF) slides stored at room temperature. J Am Acad Dermatol. 2015;73:1021-4.
- [CrossRef] [PubMed] [Google Scholar]
- Optimizing direct immunofluorescence. Methods Mol Biol. 2014;1180:111-7.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic evaluation of the lupus band test in discoid and systemic lupus erythematosus. Int J Dermatol. 1995;34:170-3.
- [CrossRef] [PubMed] [Google Scholar]
- Lupus band test for diagnostic evaluation in systemic lupus erythematosus. Lupus. 2022;31:363-6.
- [CrossRef] [PubMed] [Google Scholar]
- Role of direct immunofluorescence in cutaneous small-vessel vasculitis: Experience from a tertiary center. Am J Dermatopathol. 2018;40:661-6.
- [CrossRef] [PubMed] [Google Scholar]
- Direct immunofluorescence in cutaneous vasculitis: experience from a referral hospital in India. Indian J Dermatol. 2013;58:22-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Role of direct immunofluorescence in dermatological disorders. Indian Dermatol Online J. 2015;6:172-80.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comparison of direct immunofluorescence of plucked hair and skin for evaluation of immunological remission in pemphigus. Indian Dermatol Online J. 2017;8:319-22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An introduction to performing immunofluorescence staining. Methods Mol Biol. 2019;1897:299-311.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dew drops on spider web appearance: A newly named pattern of IgG4 deposition in pemphigus with direct immunofluorescence. Postepy Dermatol Alergol. 2017;34:295-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diagnostic value and practicability of serration pattern analysis by direct immunofluorescence microscopy in pemphigoid diseases. Acta Derm Venereol. 2021;101:adv00410.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Serration pattern analysis for differentiating epidermolysis bullosa acquisita from other pemphigoid diseases. J Am Acad Dermatol. 2018;78:754-9.
- [CrossRef] [PubMed] [Google Scholar]
- Accuracy of serration pattern analysis by direct immunofluorescence in subepidermal autoimmune blistering diseases. Indian J Dermatol Venereol Leprol 2023:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of clinical and laboratory use of the cutaneous direct immunofluorescence assay. JAMA Dermatol. 2021;157:1343-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Direct immunofluorescence using non-lesional buccal mucosa in mucous membrane pemphigoid. Front Med (Lausanne). 2018;5:20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Updated S2K guidelines on the management of pemphigus vulgaris and foliaceus initiated by the european academy of dermatology and venereology (EADV) J Eur Acad Dermatol Venereol. 2020;34:1900-13.
- [CrossRef] [PubMed] [Google Scholar]
- Paraneoplastic pemphigus: Revised diagnostic criteria based on literature analysis. J Cutan Pathol. 2021;48:1133-8.
- [CrossRef] [PubMed] [Google Scholar]
- Updated S2 K guidelines for the management of bullous pemphigoid initiated by the european academy of dermatology and venereology (EADV) J Eur Acad Dermatol Venereol. 2022;36:1689-1704.
- [CrossRef] [PubMed] [Google Scholar]
- Bullous pemphigoid in India: Review of cases registered in an autoimmune bullous disease clinic. Indian J Dermatol Venereol Leprol. 2023;89:553-7.
- [CrossRef] [PubMed] [Google Scholar]
- A retrospective study of dermatitis herpetiformis from an immunobullous disease clinic in north India. Int J Dermatol. 2018;57:959-64.
- [CrossRef] [PubMed] [Google Scholar]
- An update on direct immunofluorescence for diagnosing dermatitis herpetiformis. Postepy Dermatol Alergol. 2019;36:655-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dermatitis herpetiformis: An update on diagnosis, disease monitoring, and management. medicina (Kaunas). 2021;57:843.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






