Translate this page into:
Disinfection, sterilization and operation theater guidelines for dermatosurgical practitioners in India
2 Dermatologist, Central Govt. Health Scheme, Ministry of Health & Family Welfare, Pune, India
Correspondence Address:
Narendra Patwardhan
Dermatologist, Shreeyash Hospital, Pune - 411 004
India
| How to cite this article: Patwardhan N, Kelkar U. Disinfection, sterilization and operation theater guidelines for dermatosurgical practitioners in India. Indian J Dermatol Venereol Leprol 2011;77:83-93 |
Abstract
Modern day dermatologists conduct different esthetic and surgical procedures, with risk of infective complications. Hence, infection control practices need to be established in dermatological practice to minimize the risk of exogenous infections. These practices include hand washing, cleaning, sterilization, disinfection, operation theater sterilization and specifications. Proper hand washing after examination of each patient and prior to any surgery with a formulation containing alcohol alone or as a combination with other agents reduces the chances of transferring infections to and from patients. Sterilization and disinfection constitute the most important aspect of infection control. Disinfectants and disinfecting procedures vary according to the environment and equipment. Proper knowledge of different processes/agents for sterilization and disinfection is essential. Disinfectants for use in hospitals should always be freshly prepared and should be of adequate strength. Sterilization is carried out most commonly using steam sterilizers or ethylene dioxide sterilizers. The waste generated during practice is a potential source of nosocomial infections and should be treated as per the proper protocol and guidelines. Trained staff to carry out these practices is essential.Introduction
Dermatological practice involves procedures that are performed in outpatient settings as well as those performed exclusively in operation theater. In dermatological surgery, adverse events are not common, but the potential for serious adverse events do exist. The rates of postoperative infections have been documented to range from 0.2% to 3.5%. [1],[2],[3] Superficial suppuration constitutes the commonest form of infections encountered, systemic infective complications being rare. [2]
Postoperative skin infections occur essentially as a result of noncompliance or breach in the standard infection control practices and lead to morbidity, disfigurement and dissatisfaction amongst patients undergoing dermatological surgery. Since many of the procedures are cosmetic procedures, such incidents may also lead to medicolegal consequences. Maintenance of strict asepsis is therefore essential.
Objectives and Scope for the Guidelines
Surgeries conducted with adequate protocols and checklists have resulted in reduction of the postoperative events and complications as demonstrated in the "Safe Surgery Saves Lives" initiative of World Health Organization (WHO). [4],[5] The guidelines outlined here are framed to establish standard guidelines about infection control for practicing dermatologists. Dermatosurgery is an evolving, but rapidly expanding, subspeciality. More and more dermatologists now practice dermatosurgery. However, there is little formal teaching of different sterilization and disinfection procedures during postgraduation in dermatology. There is therefore a need for dermatosurgeons to acquire knowledge about infection control practices. This article reviews the different aspects of this topic and suggests minimum standards and practices that need to be established and followed in dermatosurgery practice. The guidelines have to be read and practiced in coordination with other guidelines for laser theater, dermatosurgery theater, and individual dermatosurgical techniques which have been previously published by dermatosurgery taskforce of Indian Association of Dermatologists, Venereologists and Leprologists (IADVL). It is also emphasized that manufacturers should always be consulted for any specific instruction for sterilization/disinfection of a specific equipment.
Definitions and Basic Principles
Infection control practices followed in dermatological practice essentially consist of cleaning, disinfection and sterilization of equipments and environment, hand hygiene practices, preoperative preparation of patient and antibiotic prophylaxis for procedures. Cleaning, disinfection and sterilization are essential to ensure that medical and surgical equipments do not transmit infectious pathogens to patients. Sterilization of all patient care items is not necessary and practitioners must identify primarily whether cleaning, disinfection or sterilization is indicated for a given item in use.
Cleaning is the physical removal of organic material or soil from the objects and is a prerequisite for disinfection or sterilization. It is usually done by using water with or without detergents. Generally, cleaning is designed to remove rather than to kill microorganisms. Ultrasonic cleaners are a useful addition to the available equipments. By using high-frequency, high-energy sound waves to hit the instrument/equipment, one can ensure that the proteinaceous material sticking to the instruments gets dislodged prior to cleaning. They are specially useful in cannulated equipments which are looped or have a natural curvature making them difficult to clean by traditional brushes. Use of automated washers will cut down on handling of instruments.
Sterilization is defined as a process that destroys or eliminates all forms of microbial life including spores and is carried out in hospitals and clinics by physical or chemical methods. Sterilization is intended to convey an absolute process. Steam under pressure, dry heat, ethylene oxide (ETO) gas are the common methods used, while newer methods include hydrogen peroxide gas plasma, low-temperature sterilization technologies and liquid chemicals.
Disinfection is a process that eliminates many or all pathogenic microorganisms without removal of bacterial spores. Unlike sterilization, disinfection is not sporicidal. In practice, instruments are usually disinfected by liquid chemicals. Some disinfectants may kill spores with prolonged exposure time and these are called chemical steriliants.
Preoperative prophylaxis includes antibiotic and antiviral administration prior to surgery. While antibiotics are routinely administered prior to all surgical procedures, antivirals such as acyclovir are administered prior to surgery/esthetic treatments on the face of patients who have prior history of herpes simplex episodes. These have been dealt with in detail under specific guidelines for each procedure and the reader is advised to consult these guidelines.
Background: Microbiology, Normal Bacterial Skin Flora and Pathophysiology of Cutaneous Infections
Postoperative infections can be either of endogenous or exogenous origin. Factors associated with transmission of infective material exogenously in hospitals and clinics include presence of shedders of pathogenic microorganisms amongst the hospital personnel, use of inadequately sterilized equipment, contaminated environment and grossly contaminated surfaces. [6],[7]
Normal human skin is colonized with bacteria; different areas of the body have varied bacterial flora and variable bacterial counts. Total bacterial counts on the hands of medical personnel have ranged from 3.9 × 10 4 to 4.6 × 10 6 . Bacteria recovered from the hands are divided into two categories: transient flora and resident flora.
Transient flora colonize the superficial layers of the skin and are usually acquired by health-care workers during direct contact with patients or contact with contaminated environmental surfaces within close proximity of the patient. Transient flora consist of microorganisms most frequently associated with health-care-associated infection and hence are very important. They are amenable to removal by routine hand washing and preoperative scrubbing of the patient′s operative area.
Resident flora are found in deeper layers of the skin and hence are more resistant to removal. However, resident flora (e.g., coagulase-negative staphylococci and diphtheroids) are less likely to be associated with such infections. The hands of health-care workers may become persistently colonized with pathogenic flora like Staphylococcus aureus, gram-negative bacilli, or yeast. Investigators have documented that although the number of transient and resident flora varies considerably from person to person, it is often relatively constant for any specific person. [8]
The prevalence and incidence of S. aureus carriage vary according to the population studied. In an Indian study from a community in Delhi, nasal carriage rate for S. aureus was 29.4%, and of these, 18.1% were Methicillin-resistant S. aureus (MRSA). Patients colonized with S. aureus have a higher risk of developing infections as compared to non-carriers. An important implication of this finding is that such patients are prone for risk of infection with MRSA, which may prove disastrous as these strains are resistant to multiple antibiotics. [9]
Bacteria on the hands of surgeons can cause wound infections if introduced into the operative field during surgery; rapid multiplication of bacteria occurs under surgical gloves if hands are washed with a non-antimicrobial soap. However, bacterial growth is slowed after preoperative scrubbing with an antiseptic agent. Reducing resident skin flora on the hands of the surgical team for the duration of a procedure reduces the risk of bacteria being released into the surgical field if gloves become punctured or are torn during surgery.
Hand Washing
Hand washing is therefore an important, cheap and a simple way of preventing nosocomial infections. Hand antisepsis reduces the incidence of health-care-associated infections.
Hand washing is broadly of two types: typical surgical scrub prior to surgery and routine hand scrub done after examining a patient. Surgical hand scrub should be elaborate and detailed, while hand scrub after patient examination may be casual. Decontaminating hands before having direct contact with patients is strongly recommended for implementation. It is supported by experimental, clinical, or epidemiologic studies and a strong theoretical rationale [10] (Evidence level A).
Health-care antiseptic drug products for handwash are of different categories as follows.
- Antiseptic handwash or health-care worker (HCW) handwash: An antiseptic-containing preparation designed for frequent use; it reduces the number of microorganisms on intact skin to an initial baseline level after adequate washing, rinsing, and drying; it is broad-spectrum, fast-acting, and mildly persistent. Formulations containing 60-95% alcohol alone or 50-95% when combined with limited amounts of a quarternary ammonium compound, hexachlorophene, or chlorhexidine gluconate are commonly used antiseptic handwashes.
- Surgical hand scrub: Patient′s preoperative skin preparation is a fast-acting, broad-spectrum, and persistent antiseptic-containing preparation that substantially reduces the number of microorganisms on intact skin. Chlorhexidine gluconate and iodophors are commonly used antimicrobials that fit in this category.
Products for hand washing
While choosing hand hygiene products, efficacy, irritancy potential, particularly when these products are used multiple times per shift, should be considered. The cost of hand hygiene products should not be the primary factor influencing product selection. The practice of "topping off" dispensers can lead to bacterial contamination of soap and hence adding soap to a partially empty soap dispenser is discouraged strongly [10] (Evidence level A).
Different agents are available for hand washing. These include alcohol, chlorhexidine gluconate, iodophors, triclosan, and plain soap, which are discussed below.
Alcohols
The antimicrobial activity of alcohols can be attributed to their ability to denature proteins. The alcohols are rapidly bactericidal rather than bacteriostatic against vegetative forms of bacteria; they also are tuberculocidal, fungicidal, and virucidal but do not destroy bacterial spores. Their cidal activity drops sharply when diluted below 50% concentration. Alcohol solutions containing 60-95% alcohol are the most effective, and higher concentrations are less potent because proteins are not denatured easily in the absence of water. The majority of alcohol-based hand antiseptics contain isopropanol, ethanol, n-propanol, or a combination of two of these products. Alcohols are usually used as a combination of two alcohols or along with other agents like hexachlorophene, quarternary ammonium compounds, povidone-iodine, triclosan, or chlorhexidine gluconate.
Chlorhexidine
Chlorhexidine gluconate is a commonly used agent and has been incorporated into a number of hand-hygiene preparations. It is a cationic bisbiguanide, and acts by disruption of cytoplasmic membranes, resulting in precipitation of cellular contents. Chlorhexidine has good activity against gram-positive bacteria, somewhat less activity against gram-negative bacteria and fungi, and only minimal activity against tubercle bacilli. The antimicrobial activity of chlorhexidine is only minimally affected by the presence of organic material such as blood. It has substantial residual activity, and addition of low concentrations (0.5-1.0%) of chlorhexidine to alcohol-based preparations results in greater residual activity than alcohol alone.
Iodophors
Iodine molecules rapidly penetrate the cell wall of microorganisms and inactivate cells by forming complexes with amino acids and unsaturated fatty acids, resulting in impaired protein synthesis and alteration of cell membranes. Iodine and iodophors have bactericidal activity against gram-positive, gram-negative, and certain spore-forming bacteria (e.g., clostridia and Bacillus spp.) and are active against mycobacteria, viruses, and fungi. However, in concentrations used in antiseptics, iodophors are not usually sporicidal. Further, the antimicrobial activity of iodophors can be affected by pH, temperature, exposure time, concentration of total available iodine, and the amount and type of organic and inorganic compounds present (e.g., alcohols and detergents).
Povidone-iodine 5-10% is widely used, as it is regarded as a safe and an effective antiseptic handwash. The majority of iodophor preparations used for hand hygiene contain 7.5-10% povidone-iodine.
Guidelines for Decontaminating Hands
a. Casual hand rub: During the outpatient examination of patients, it is recommended to use a hand scrub after examination of each patient.
When washing hands with soap and water, wet hands first with water, apply an amount of product recommended by the manufacturer to hands, and rub hands together vigorously for at least 15 seconds, covering all surfaces of the hands and fingers then rinse hands with water and dry thoroughly with a disposable towel.
While using an alcohol-based hand rub, apply product to palm of one hand and rub hands together, covering all surfaces of hands and fingers, until the hands are dry. The volume of product to be used depends on the product and hence the manufacturer′s recommendations may be followed.
A combination of agents may be more effective. It is advisable to use a handwash formulation containing 60-95% alcohol alone or 50-95% alcohol in combination with a quarternary ammonium compound or 50-95% alcohol in combination with a hexachlorophene or 50-95% alcohol in combination with chlorhexidine gluconate.
These agents lower the bacterial counts on skin immediately post scrub more effectively than do the other agents. The next most active agents (in order of decreasing activity) are chlorhexidine gluconate, iodophors, triclosan, and plain soap. [10] Bar soap commonly used should be kept in a dry tray. Wetness could promote growth of bacteria on soap.
Always use a disposable towel to turn off the faucet. Multiple-use cloth towels of the hanging or roll type are not recommended for use in health-care settings. [10] Avoid using hot water because repeated exposure to hot water may increase the risk of dermatitis.
b. Surgical hand scrub: Rings, watches, and bracelets should always be removed before beginning the surgical hand scrub. Debris from underneath fingernails may be removed using a nail cleaner under running water. Surgical hand antisepsis before donning sterile gloves may be done either by using either an antimicrobial soap or an alcohol-based hand rub with persistent activity (IB). When using an antimicrobial soap for surgical hand antisepsis, scrub hands and forearms for 2-6 minutes (or as recommended by the manufacturer). Longer scrub times (e.g., 10 minutes) are not necessary (IB) [Annexure 1]and [Figure - 1] and [Figure - 2].
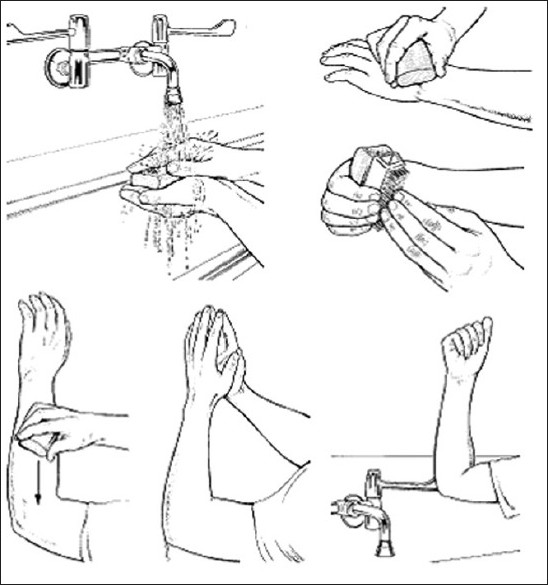 |
| Figure 1: Diagram showing handwashing technique |
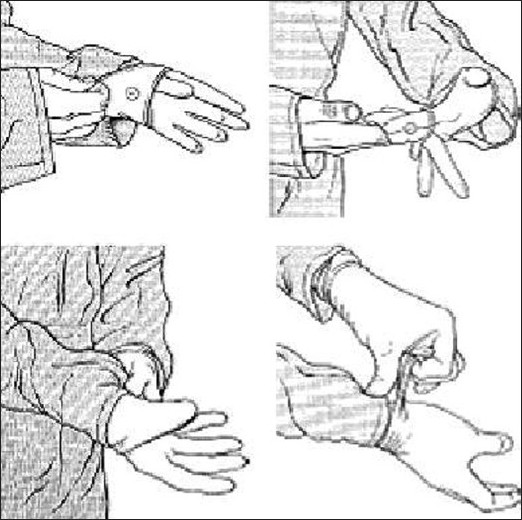 |
| Figure 2: Diagram showing technique for wearing gloves |
c. Alcohol-based surgical scrubs: With alcohol-based scrubs, thorough prewash of hands and forearms with a non-antimicrobial soap and drying hands and forearms is recommended. The hands should be dry before alcohol solution is applied. Alcohol will not work on wet hands as a disinfectant. After application of the alcohol-based product as recommended, allow hands and forearms to dry thoroughly before donning sterile gloves (IB). [10] The above method will prevent bacterial growth below the gloves for extended period of time during surgery. This can prevent contamination of the surgical field in the event of tear of gloves. Donning a sterile glove without washing the underlying hand is not recommended.
Hand Washing for Dermatosurgical Procedures
These vary with respect to the handwash type needed. For electrosurgery, microdermabrasion and suture surgery, the dermatologist may wash their hands with a disinfectant - chlorhexidine, iodine scrub lotion or alcohol. For advanced and invasive procedures such as skin resurfacing, punch grafting, hair restoration, liposuction, complex closures, thorough hand scrubbing for 2 minutes with surgical scrub up to elbow is essential.
For preparation of the skin over surgical area prior to surgery, povidine iodine 5% w/v is sufficient. But for povidine iodine to be effective, it should be left on the skin of the patient for at least 2 minutes. Alcohol should not be used when using electrosurgery unit or lasers.
Disinfection
Disinfection describes a process that eliminates most, if not all, pathogenic microorganisms, except bacterial spores. As stated earlier, unlike sterilization, disinfection is not sporicidal, though some disinfectants may kill spores with prolonged exposure time (called chemical steriliants, e.g., glutaraldehyde). Disinfectants are generally classified as follows.
- a. High level disinfectants : Chemicals that kill all microorganisms except bacterial spores, when exposed for limited time. Germicides categorized as chemical steriliants are high level disinfectants. These include 2.4% glutaraldehyde based formulations, 0.94% glutaraldehyde with 1.64% phenol, 7.5% stabilized hydrogen peroxide, 7.35% hydrogen peroxide with 0.23% peracetic acid, 0.8% peracetic acid with 1% hydrogen peroxide.
- b. Intermediate level disinfectants : They kill mycobacteria, vegetative bacteria, most viruses and most fungi but do not necessarily kill bacterial spores. Phenolics, alcohols, iodophors and chlorine-based agents act as intermediate level disinfectants.
- c. Low level disinfectants : Can kill most vegetative bacteria, some fungi and some viruses. Commonly used detergents and soaps act as low level disinfectants along with their cleaning properties.
Instruments and different types of disinfection
The precise type of disinfection depends on the type of instruments. Medical devices, equipment, and surgical materials are divided into three general categories depending on the potential risk of infection involved in their use as recommended by Spaulding: [11] critical items, semicritical items, and non-critical items.
- Critical items : These are instruments or objects that are introduced directly into the bloodstream or into other normally sterile areas of the body. Examples of critical items are surgical instruments, cardiac catheters, implants, pertinent components of the heart-lung oxygenator, and the blood compartment of a hemo-dialyzer. Absolute sterility at the time of use is required for these items; consequently, one of the several accepted sterilization procedures is generally recommended. Dermatosurgical instruments involved in hair transplantation, liposuction, vitiligo surgery, some forms of acne surgery, surgical excisions and scar revisions belong to this category.
- Semicritical items : These instruments are introduced into body cavities and therefore come into contact with intact mucous membranes, but do not ordinarily penetrate body surfaces. Examples for such instruments include noninvasive flexible and rigid fiber-optic endoscopes, endotracheal tubes, anesthesia breathing circuits, and cystoscopes. A high-level disinfection procedure (which can destroy vegetative microorganisms, most fungal spores, tubercle bacilli, and small non-lipid viruses) with prior meticulous physical cleaning is adequate in most situations. Amongst the chemical steriliants, the most commonly employed for this purpose is glutaraldehyde. Glutaraldehyde solution should be alkaline and of more than 2% strength. The activated glutaraldehyde solution can be used for about a fortnight. The equipment should be kept immersed in glutaraldehyde solution for at least 45 minutes. If feasible, steam sterilization, may be used to sterilize many of these items, but is not absolutely essential.
- Non-critical items: These are items that do not ordinarily penetrate, but touch only intact skin. Such items include crutches, bed boards, blood pressure cuffs, and a variety of other medical accessories. These items rarely, if ever, transmit disease. Many dermatological instruments such as microdermabrasion tips, laser probes, laser glasses, phototherapy glasses may be classified as non-critical items. Consequently, depending on the particular piece of equipment or item, simple washing with a detergent may be sufficient.
Types of disinfectants
The different types of disinfectants available for use are shown in [Table - 1]. They essentially belong to the following broad groups: alcohols, aldehydes (such as formaldehyde, glutaraldehyde, ortho-phthalaldehyde), oxidizing agents (such as chlorine and chlorine releasing compounds), hydrogen peroxide, peracetic acid, phenolics and quarternary ammonium compounds. Ultraviolet radiation and pasteurization are also sometimes employed for disinfection.
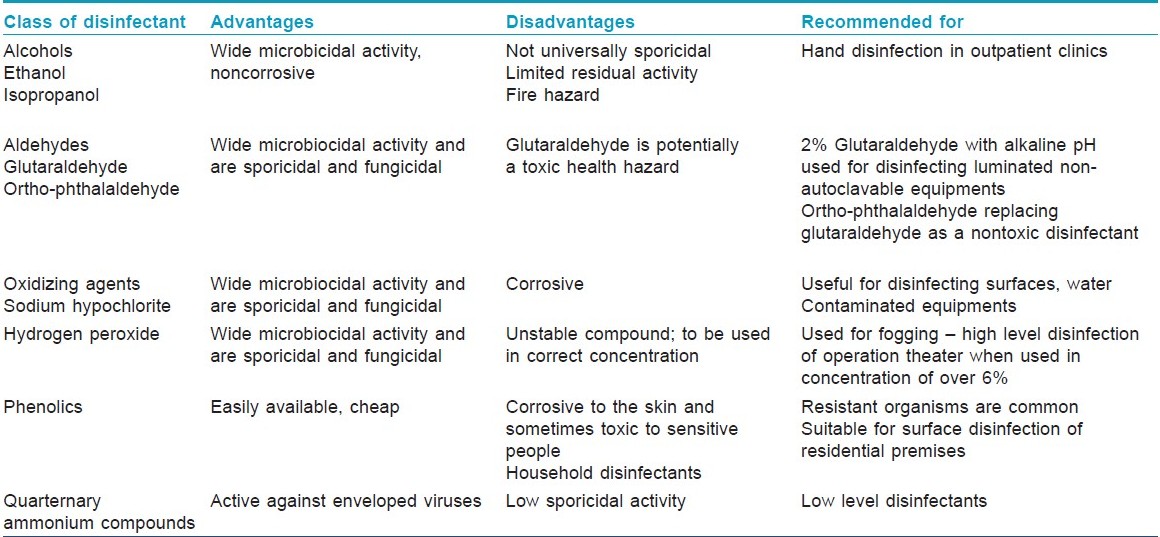
Disinfectants for use in hospitals should always be freshly prepared and should be of adequate strength. Nosocomial infections due to usage of contaminated antiseptics have been reported on numerous occasions. In a study carried out in Pune, antiseptic solutions in a large general hospital were found to be contaminated with pathogenic bacteria including Pseudomonas aeruginosa and Klebsiella species. [12]
Sterilization
Most important methods for sterilization of instruments include steam sterilization (autoclaving) and ethylene dioxide gas sterilization.
Steam sterilization is the most commonly used method for sterilization of instruments and gadgets, as it is a nontoxic and an inexpensive system requiring relatively shorter sterilization time. Adequate autoclaving using steam displacement method requires a holding time of 10 minutes at 121°C at a pressure of 15 lbs or of 3 minutes at 134°C at a pressure of 20 lbs. It is recommended that all equipments which can withstand the effects of temperature should be sterilized by steam sterilization. The autoclaving process needs periodic monitoring. Chemical indicators are incorporated regularly with autoclaving cycles. Single parameter chemical indicators indicate attainment of a certain temperature by a visible color change. Multiparameter chemical indicators called as "integrators" indicating temperature, pressure and time are also available. Biological indicators need to be used periodically to substantiate the process of sterilization, especially in developing countries like India. [13]
ETO gas sterilization is a more complex and expensive process than steam sterilization. Hence, it is usually restricted to objects that might be damaged by heat or excessive moisture. Before gas sterilization, objects need to be cleaned thoroughly and wrapped in a material that allows the gas to penetrate. Chemical indicators need to be used with each package to show that it has been exposed to the gas sterilization process. Moreover, it is recommended that gas sterilizers be checked at least once a week with commercial preparations of spores, usually Bacillus subtilis var. niger. Because ETO gas is toxic, precautions (e.g., local exhaust ventilation) should be taken to protect personnel. All objects processed by gas sterilization also need special aeration according to manufacturer′s recommendations before use to remove toxic residues of ETO. ETO sterilizers are widely used in hospitals for sterilizing heat sensitive equipments. Sterilized products also have a longer shelf-life when they are processed by ETO sterilizers. Small ETO sterilizers are also available which can be used in clinics.
Other less commonly used methods include dry heat which can be used to sterilize powders and anhydrous oils. Hydrogen peroxide gas plasma and low temperature sterilization technologies are newer methods of sterilization available recently.
Design and Disinfection of Operation Theater
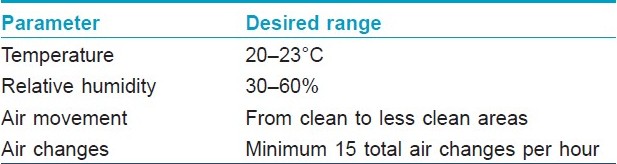
Detailed guidelines about dermatosurgery operation theater have previously been published and only a brief mention is made here. Dermatosurgery theaters should adhere to the definition of a clean room which is defined as a room in which the concentration of airborne particles is controlled, and which is constructed and used in a manner to minimize the introduction, generation, and retention of particles inside the room and in which other relevant parameters, e.g., temperature, humidity, and pressure, are controlled as necessary. Operation theaters carrying out dermatological surgeries require adhering to certain physical and microbiological parameters so as to ensure that the chances of exogenous postoperative infections are minimized. Physical parameters for an operation theater are summarized in [Table - 2]. The ventilation requirements for operating rooms are an outward air movement in relation to the adjacent area, a minimum of 15 total air changes per hour, a relative humidity of 30-60% and a design temperature of 20-23°C. The use of laminar air flow systems is advised in operation theaters but their value has not been documented for all types of theaters, [14] and therefore, is not recommended for dermatosurgical theaters.
Disinfection of operation theater
It should be emphasized that general operation theater layouts, operating room etiquette, sterilization of instruments, sterile surgical protocol are all highly important and relevant factors, which directly affect the incidence of postoperative infections in any setting. Proper protocols need to be put in place for proper adoption of these simple procedures by all theater staff.
Aldehydes are the most commonly used agents for high level disinfection of the theater environment. Formaldehyde is the commonly used agent. Formaldehyde gas is generated from liquid formalin utilizing potassium permanganate crystals. 40% formalin liquid is added to potassium permanganate crystals to generate gas. Alternately, formalin liquid can be dispersed by a sprayer like device in the theater environment. After a contact time of at least 6-8 hours, the formaldehyde needs to be neutralized by using ammonia, allowing at least 2 hours contact time for ammonia to neutralize the formaldehyde prior to the use of theater. Aldehydes are potentially carcinogenic and it is therefore recommended that other agents such as hydrogen peroxide, hydrogen peroxide with silver nitrate, peracetic acid and other chemical compounds of formaldehyde [Table - 3] should be used in place of the currently prevalent practice of using formaldehyde. These agents are dispersed with the aid of a fogger-like device inside the theater environment. The contact time is about an hour and the theater can be used immediately after the contact time.

Other agents used as high level disinfectants through a fogger are hydrogen peroxide, hydrogen peroxide along with silver nitrate, peracetic acid and other chemical compounds of formaldehyde [Table - 3].
The inanimate surfaces in the operation theater need proper periodic cleaning and disinfection. Grossly contaminated surfaces are the potential sources of hospital acquired infections. The operation theater should be thoroughly washed with water and detergent mixture so as to get rid of all spills and proteinaceous materials before a disinfectant is used for mopping the surfaces.
It has been observed that there is a general relationship between the aerobic bacterial count and the risk of infection. There is a significant risk of infection with counts in the range of 700-1800 bacteria carrying particles (BCP) per cubic meter. However, the risk is slight when the BCP load is less than 180/m 3 . [15] Surveillance of the air conditioning (AC) units, the in-use disinfectants and the personnel working in close vicinity during operative procedures needs to be incorporated as a periodic monitoring tool to be used judiciously. [7]
Hospital acquired infections are usually due to opportunistic fungi like Aspergillus, Rhizopus, Fusarium and Penicillium.[16] All of these are capable of proliferating in AC units. The filters utilized in these units can act as suitable nidus for growth and proliferation of the pathogenic fungi, the spores of which can be discharged and carried through the theater air. Presence of fungi in AC units and subsequent dispersal of spores could be a potential hazard, especially for the immunocompromised patients. The operating room and clinic AC units should be meticulously maintained and frequently monitored to minimize the chances of growth and proliferation of potentially pathogenic fungi. Split AC units appear to be a safer option than the window mounted AC units. [17]
S. aureus and β hemolytic streptococci are important health-care associated pathogens and can persist in the environment for extended periods. They can be shed and can lead to infections, when health-care personnel are heavily colonized with these organisms. S. aureus and b hemolytic streptococci have been linked to airborne transmission in operation theaters, burn units, and neonatal units. [18],[19] Periodic screening, treatment of shedders and quarantining them from operations till the treatment is completed, would ensure that the risk of transmission of infections is minimized. [7]
It needs to be recognized here that dermatosurgical procedures are usually noninvasive, performed under local anesthesia and often with disposable instruments, in usually healthy patients. Hence, any disinfection/sterilization of instruments in dermatosurgery needs the category of instruments and the type of procedure to be considered. The dermatological procedures routinely carried out in clinics and hospitals along with the recommended disinfection/sterilization methods advocated are summarized in [Table - 4].
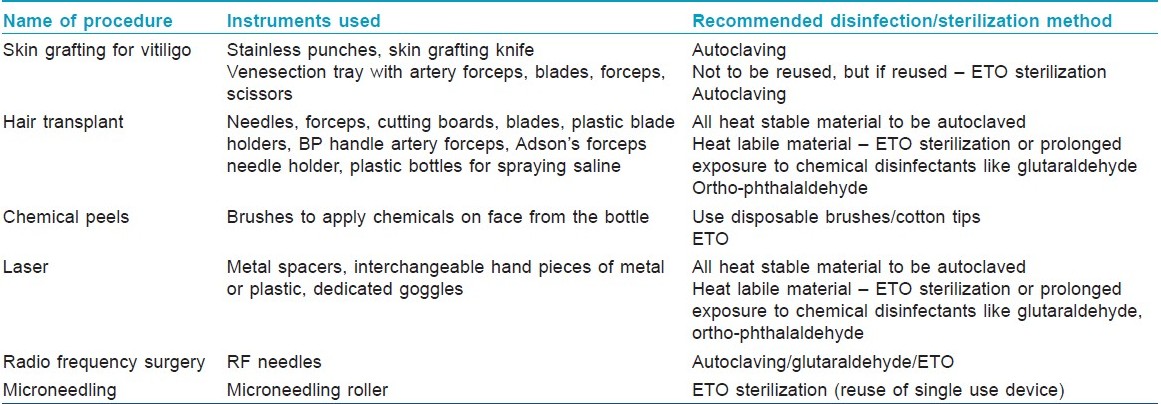
Waste Disposal
Infectious waste constitutes soiled surgical dressings, swabs, material in contact with infectious diseases and sharps. The waste generated has to be segregated at source as early as possible. Waste disposal is an important aspect of health care and strict laws have been enacted for proper waste disposal and environmental safety. The management of this waste is guided by the rule of Central Pollution Control Board, Ministry of environment, Government of India, which calls for mandatory registration with appropriate authorities for pollution control and waste disposal. Specific instructions may vary from state to state and the physician is advised to consult the concerned authorities in their respective states.
All clinics need to register with the concerned authorities and engage agencies for waste disposal. Different colored bags for disposal of waste and sharps should be available and the color codes should be displayed prominently. Concerned staff should be properly trained in waste disposal. The waste should be disinfected prior to disposal.
There are two aspects of management:
- segregation of the biomedical waste and
- safe disposal of the biomedical waste.
Staff should segregate the waste at source into leak proof and puncture proof high density plastic container with polythene bag inside for proper disposal of the generated waste. The biohazard logo should be on the container and bag. Later, this can be handed over to either hospital waste management team, or individual dermatologists in remote areas and peripheries can organize their safe disposal as per the suggestions below.
Dermatology clinics generate three types of biomedical waste. [20] [Table - 5] shows the different types of waste and their recommended practices for waste disposal.

Annexure 1 gives the guidelines for handwash, gloves, masks, gowns and foot wear.
Conclusion
As dermatosurgeons carry out different invasive procedures, disinfection for dermatosurgery instruments is of utmost necessity. It needs to be carried out as per the established procedures and guidelines to ensure patient safety.
| 1. |
Amici JM, Rogues AM, Lasheras A, Gachie JP, Guillot P, Beylot C, et al. A prospective study of the incidence of complications associated with dermatological surgery. Br J Dermatol 2005;153:967-71.
[Google Scholar]
|
| 2. |
Chan BC, Patel DC. Perioperative management and the associated rate of adverse events in dermatological procedures performed by dermatologists in New Zealand. Australas J Dermatol 2009;50:23-8.
[Google Scholar]
|
| 3. |
Salanitri S, Gonçalves AJ, Helene A Jr, Lopes FH. Surgical complications in hair transplantation: A series of 533 procedures. Aesthet Surg J 2009;29:72-6.
[Google Scholar]
|
| 4. |
Tools and resources. WHO - Safe surgery saves lives. c2009. Available from: http://www.who.int/patientsafety/safesurgery/en/ [last cited on 2009 Apr 29].
[Google Scholar]
|
| 5. |
Mysore V, Anitha B. Checklists for surgical safety in dermatosurgery. J Cutan Aesthet Surg 2009;2:1-3.
[Google Scholar]
|
| 6. |
Ram J, Kaushik S, Brar GS, Taneja N, Gupta A. Prevention of postoperative infections in ophthalmic surgery. Indian J Ophthalmol 2001;49:59-69.
[Google Scholar]
|
| 7. |
Kelkar U, Kelkar S, Bal AM, Kulkarni S, Kulkarni S. Microbiological evaluation of various parameters in ophthalmic operating rooms. The need to establish guidelines. Indian J Ophthalmol 2003;51:171-6.
[Google Scholar]
|
| 8. |
Sprunt K, Redman W, Leidy G. Antibacterial effectiveness of routine hand washing. Pediatrics 1973;52:264-71.
[Google Scholar]
|
| 9. |
Saxena S, Singh K, Talwar V. Methicillin resistant Staphylococcus aureus prevalence in community in the east Delhi area. Jpn J Infect Dis 2003;56:54-6.
[Google Scholar]
|
| 10. |
Boyce JM, Pittet D; Healthcare Infection Control Practices Advisory Committee; HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm Rep 2002;51:1-45.
[Google Scholar]
|
| 11. |
Favero MS. Chemical disinfection of medical and surgical materials. In: Block SS, editor. Disinfection, sterilization and preservation. 3 rd ed. Philadelphia: Lea and Febiger; 1983. p. 469-92.
[Google Scholar]
|
| 12. |
Arjunwadkar VP, Bal AM, Joshi SA, Kagal AS, Bharadwaj RS. Contaminated antiseptics- an unnecessary hospital hazard. Indian J Med Sci 2001;55:393-8.
[Google Scholar]
|
| 13. |
Kelkar U, Bal AM, Kulkarni S. Monitoring of steam sterilization process by biological indicators- a necessary surveillance tool for developing countries. Am J Infect Control 2004;32:512-3.
[Google Scholar]
|
| 14. |
Centres for Disease Control and Prevention, Health care infection control practices advisory committee, Draft guideline for environmental infection control in health care facilities, Appendix G. 2001. p. 197.
[Google Scholar]
|
| 15. |
Parker MT. World Health Organization. Hospital acquired infections: Guidelines to laboratory methods. Copenhagen: WHO Regional Publications European Series No. 4; 1978. p. 28-33.
[Google Scholar]
|
| 16. |
Overberger PA, Wadowsky RM, Schaper MM. Evaluation of air-borne particulates and fungi during hospital renovation. Am Ind Hyg Assoc J 1995;56:706-12.
[Google Scholar]
|
| 17. |
Kelkar U, Bal AM, Kulkarni S. Fungal contamination of air conditioning units in operating theaters in India. J Hosp Infect 2005;60:81-4.
[Google Scholar]
|
| 18. |
Wenzel RP, Veazey JM Jr, Townsend TR. Role of inanimate environment in hospital-acquired infections. In: Cundy KR, Ball W, editors. Infection control in healthcare facilities: Microbiological surveillance. Baltimore: University Park Press; 1977. p. 71-98.
[Google Scholar]
|
| 19. |
Mortimer EA Jr, Wolinsky E, Gonzaga AJ, Rammelkamp CH Jr. Role of airborne transmission in staphylococcal infections. Br Med J 1966;1:319-22.
[Google Scholar]
|
| 20. |
Annonymous. Categories of biomedical waste. Schedule -I and Schedule II. Government of India: Ministry of Environment and Forest Gazette Notification; 2000.
[Google Scholar]
|
Fulltext Views
73,251
PDF downloads
6,903





