Translate this page into:
Disseminated cutaneous Mycobacterium haemophilum infection in an immunocompromised Chinese patient presenting with multifocal nodules
2 Center for Pathogen Research, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Correspondence Address:
Lin Wang
Department of Dermatovenereology, West China Hospital, Sichuan University, 37# Guoxue Alley, Wuhou District, Chengdu, Sichuan 610041
China
| How to cite this article: Li Z, Wang X, Ran Y, Wang L. Disseminated cutaneous Mycobacterium haemophilum infection in an immunocompromised Chinese patient presenting with multifocal nodules. Indian J Dermatol Venereol Leprol 2020;86:181-184 |
Sir,
Mycobacterium haemophilum, a strongly acid-fast bacillus (AFB) belonging to the group of slow growing nontuberculous mycobacteria, was first isolated in 1978 and is known to cause skin infections most frequently in immunocompromised patients.[1] Cutaneous manifestations, including erythematous nodules, papules, plaques, ulcers and abscesses, are the most common presentations usually localized to the extremities.[2] To date, disseminated cutaneous M. haemophilum infection presenting with multiple nodules covering the entire body has rarely been reported. 3 Line arrangement of bacteria observed with acid-fast staining described in this report was seen even rarer, with only a few cases reported.[1]
A 51-year-old woman, a Chinese peasant, presented to our outpatient clinic with an 8-month history of painful cutaneous nodules. Initially, she noticed reddish violet nodules on her heels, which gradually spread to the face over a period of 8 months. The patient denied the presence of fever, chills, cough or fatigue. She gave a history of cryptococcal meningitis in the previous year which was treated with amphotericin B, fluconazole, flucytosine, and methylprednisolone. The patient had taken dexamethasone 0.75–1.5 mg/day on her own for a duration of one year prior to the onset of cryptococcal meningitis. After the antifungal therapy, she continued using the same dose of dexamethasone for one more year. Physical examination revealed disseminated reddish violet nodules, plaques and macules varying in size from 0.5 cm to 2 cm seen all over her body [Figure - 1]a and [Figure - 1]b.
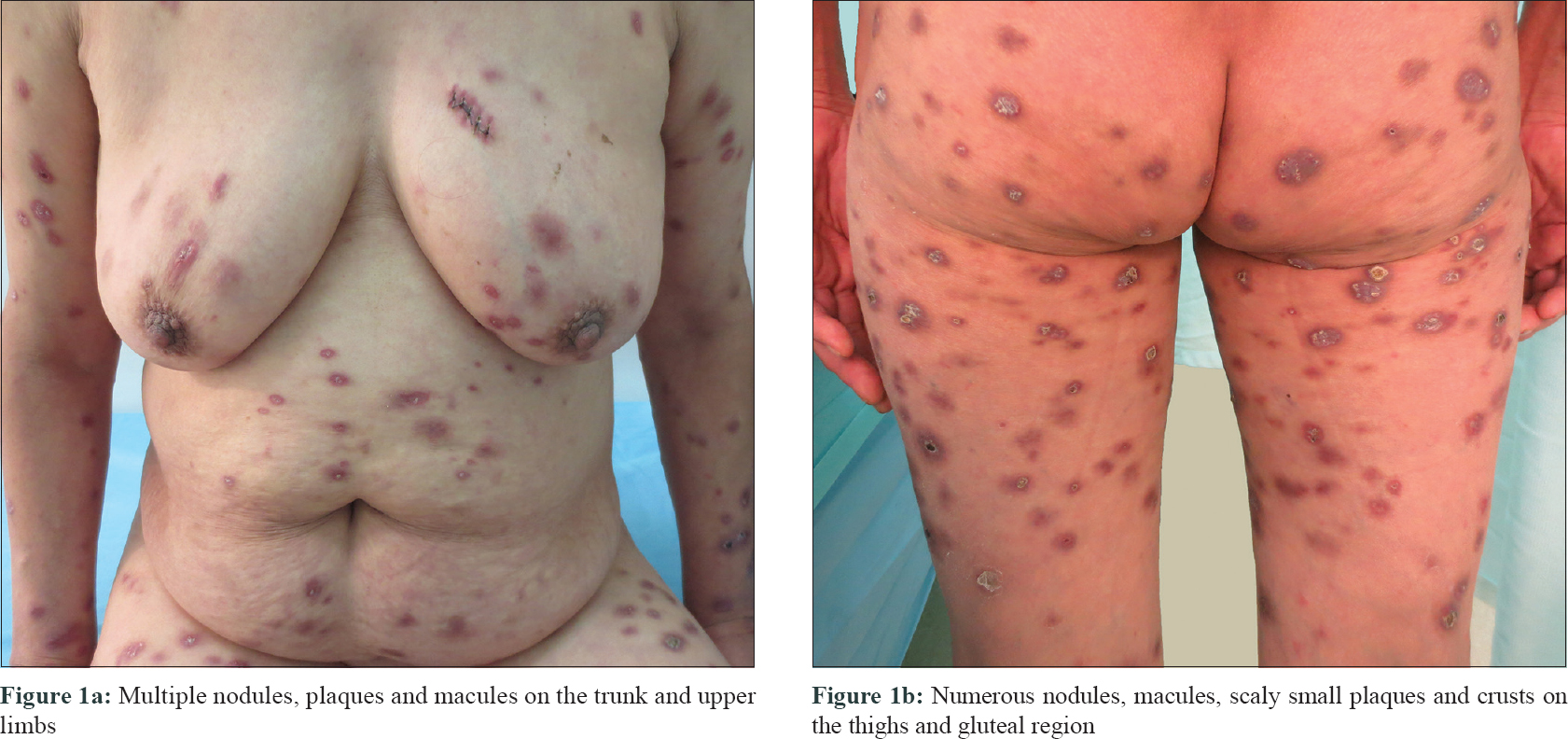 |
| Figure 1: |
The erythrocyte sedimentation rate was elevated with a level of 44 mm in the 1st hour. The immunological profile displayed a dominant decrease in CD3, CD4, and CD8 cells. The liver function test revealed an elevated gamma-glutamyl transpeptidase level of 264 IU/L and an increased alanine aminotransferase level of 58 IU/L. The purified protein derivative test showed a negative result. The serological test for human immunodeficiency virus, (1,3)-β-D-glucan assay was also negative and no abnormal findings could be detected in X-ray of chest. Skin biopsies taken from the chest and waist showed perivascular lymphocytes and focal dense neutrophils in the upper dermis. In the lower dermis, sheets of epithelioid histiocytes and some lymphocytes and plasmacytes were observed [Figure - 2]a. On immunohistochemistry, histiocytes expressed CD68 [Figure - 2]b and the lymphocytes expressed CD3ε indicating mature T cells. Scattered CD138+ cells (plasma cells) were also observed. The Ki-67 proliferation index was approximately 5.0% [Figure - 2]c. Acid-fast staining revealed numerous bacteria arranged in clusters and lines [Figure - 2]d. Transmission electron microscopy revealed rod-shaped and sheathed bacteria [Figure - 3]. To identify the species of mycobacteria, the tissue was subjected to polymerase chain reaction (PCR) to amplify a 422-bp fragment of the heat-shock protein gene (hsp65), using the primers 5-′-ATCGCCAAGGAGATCGAGCT-3-′ (forward) and 5-′-AAGGTGCCGCGGATCTTGTT-3-′ (reverse) and the PCR product was directly sequenced.[4] The sequence was in accordance with Mycobacterium haemophilum (GenBank Accession No. NZ_CP011883.2) with homology 99% using the Blast sequences tool. However, tissue culture for M. haemophilum on a chocolate agar medium supplemented with ferric ammonium citrate after 8 weeks of incubation at 32° was negative. Hence, a final diagnosis of disseminated cutaneous M. haemophilum infection was established based on PCR as well as clinical and laboratory findings. To reduce the hepatotoxicity caused by drugs, an oral regimen consisting of rifampin (450 mg once a day) and ciprofloxacin (500 mg twice a day) was started. After one month of therapy, the nodular lesions faded. As there were no new skin lesions, patient stopped the treatment on her own.
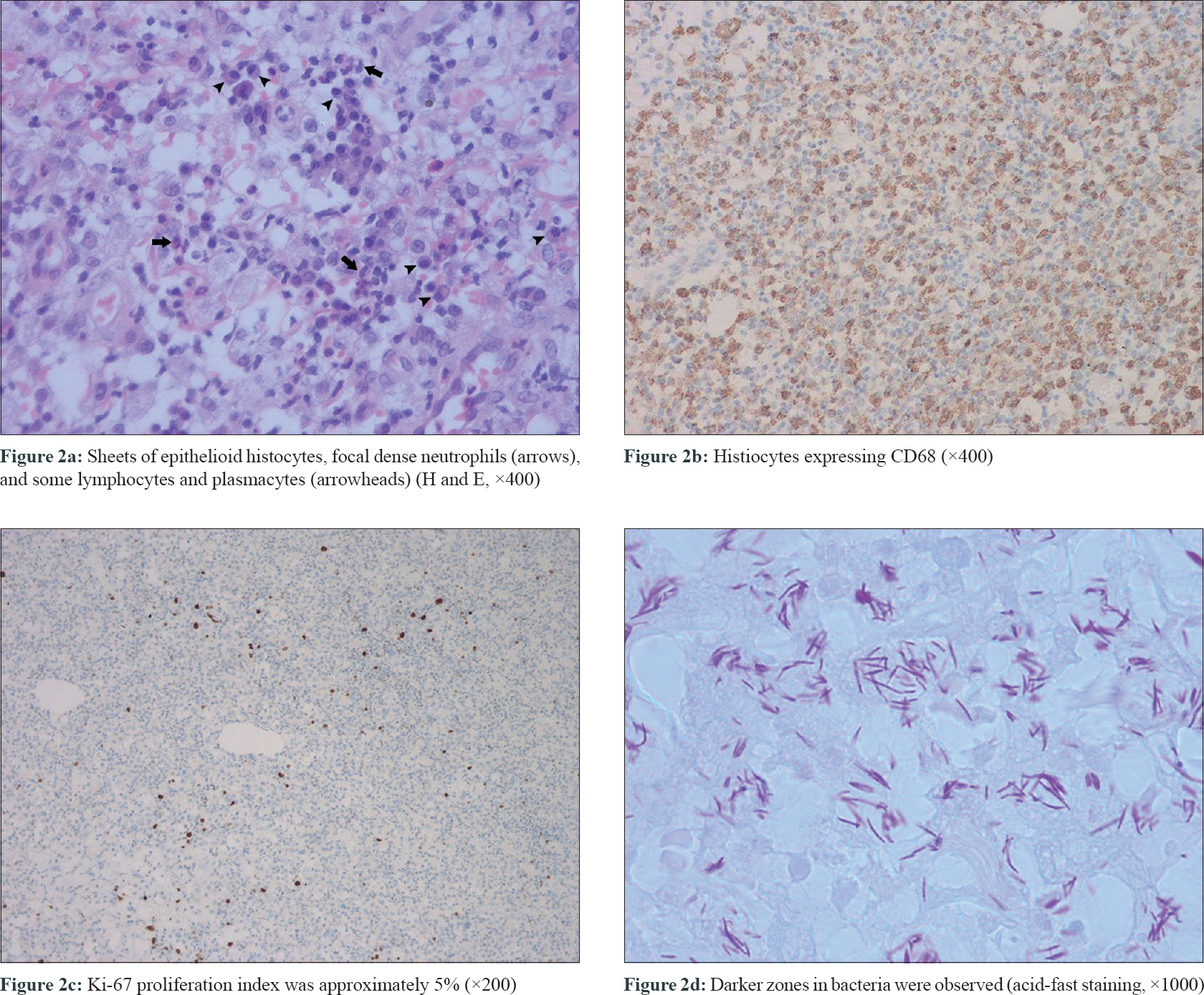 |
| Figure 2: |
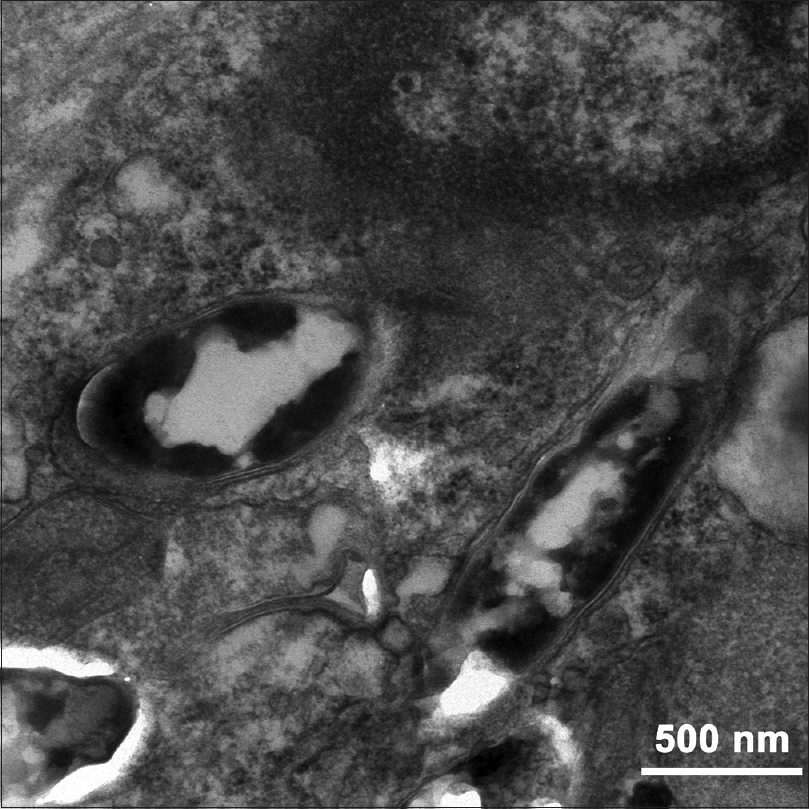 |
| Figure 3: Rod-shaped and sheathed bacteria (transmission electron microscopy) |
M. haemophilum infects mostly immunocompromised individuals and presents an array of symptoms ranging from focal involvement to widespread disease, including septic arthritis, osteomyelitis, pneumonitis and lymphadenitis.[5] Cutaneouslesions of M. haemophilum infection are usually located at cooler body sites such as extremities sometimes in a sporotrichoid pattern owing to the low-temperature requirements of M. haemophilum for maximal growth.[6],[7] But our patient had lesions not only in the extremities but also on the trunk and face. Long term inadvertent systemic use of glucocorticoids might have resulted in her immunocompromised condition thereby increasing her susceptibility to pathogens and her habit of going barefoot on the farm would have increased her exposure to diverse pathogens in the environment.
The most common histological pattern seen in M. haemophilum infection is a mixed suppurative and granulomatous reaction.[8] Lack of a pronounced granulomatous reaction, which can occur in immunocompromised patients, can sometimes misguide dermatopathologists. The rigorous conditions needed for the culture of M. haemophilum often lead to a negative result which also challenges the diagnosis.[9] M. haemophilum appears as short and often curved rods of 1.2–2.5 μm length.[2] But in this patient acid-fast staining showed longer bacilli of >5.0 μm, which we presume may be due to the specific overlapping pattern of M. haemophilum in the tissue. The darker zone in a bacillus may be the overlapping part of two bacilli in line arrangement, indicating that the bacilli tend to be arranged end to end leading to the phenomenon of a longer bacillus in light microscopy. This specific bacterial arrangement pattern in acid-fast staining, which was not found in other mycobacteria, could be an important predictor in the diagnosis of M. haemophilum infection.
Standard guidelines are not yet available for the treatment of M. haemophilum. Experts have recommended treatment with multiple antibiotics (e.g. clarithromycin, ciprofloxacin and rifampin) for a duration of 1 to 2 years.[3] In this case, the patient was administered ciprofloxacin and rifampin and exhibited partial improvement after 1 month.
In conclusion, we report a rare case of cutaneous M. haemophilum infection in the Chinese population. Immunocompromised patients presenting with atypical disseminated lesions should raise a high suspicion of atypical mycobacterial infection. It is necessary to perform acid-fast staining and PCR sequence analysis to diagnose such infections.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Acknowledgement
The authors would like to thank Min Zou from Guangyuan Central Hospital for helping them collect clinical data of the patient.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Sompolinsky D, Lagziel A, Naveh D, Yankilevitz T. Mycobacterium haemophilum sp. nov., a new pathogen of humans. Int J Syst Bacteriol 1978;28:67-75.
[Google Scholar]
|
| 2. |
Lindeboom JA, Bruijnesteijn van Coppenraet LE, van Soolingen D, Prins JM, Kuijper EJ. Clinical manifestations, diagnosis, and treatment of Mycobacterium haemophilum infections. Clin Microbiol Rev 2011;24:701-17.
[Google Scholar]
|
| 3. |
Rajpara A, Sri J, Driscoll M. Mycobacterium haemophilum: Cutaneous nodules in a renal transplant patient. Dermatol Online J 2010;16:3.
[Google Scholar]
|
| 4. |
Kim H, Kim SH, Shim TS, Kim MN, Bai GH, Park YG, et al. Differentiation of Mycobacterium species by analysis of the heat-shock protein 65 gene (hsp65). Int J Syst Evol Microbiol 2005;55:1649-56.
[Google Scholar]
|
| 5. |
Saubolle MA, Kiehn TE, White MH, Rudinsky MF, Armstrong D. Mycobacterium haemophilum: Microbiology and expanding clinical and geographic spectra of disease in humans. Clin Microbiol Rev 1996;9:435-47.
[Google Scholar]
|
| 6. |
Shah MK, Sebti A, Kiehn TE, Massarella SA, Sepkowitz KA. Mycobacterium haemophilum in immunocompromised patients. Clin Infect Dis 2001;33:330-7.
[Google Scholar]
|
| 7. |
Gao W, Chen H, Wang H, Jiang H, Liu W, Hao D, et al. Bilateral sporotrichoid cutaneous infection by Mycobacterium haemophilum in a Chinese patient with systemic lupus erythematosus. Acta Derm Venereol 2015;95:760-1.
[Google Scholar]
|
| 8. |
Busam KJ, Kiehn TE, Salob SP, Myskowski PL. Histologic reactions to cutaneous infections by Mycobacterium haemophilum. Am J Surg Pathol 1999;23:1379-85.
[Google Scholar]
|
| 9. |
Lott JP, Werth VP, Kovarik CL. Cutaneous Mycobacterium haemophilum infection in iatrogenically immunocompromised patients without transplantation. J Am Acad Dermatol 2008;59:139-42.
[Google Scholar]
|
Fulltext Views
3,500
PDF downloads
2,668





