Translate this page into:
Disseminated herpes simplex virus and varicella zoster virus co-infection in an immunocompetent patient
2 National Skin Centre; Department of Dermatology, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore,
Correspondence Address:
Ziying Vanessa Lim
1 Mandalay Road, Singapore 308205
| How to cite this article: Lim ZV, Tey H. Disseminated herpes simplex virus and varicella zoster virus co-infection in an immunocompetent patient. Indian J Dermatol Venereol Leprol 2018;84:212-214 |
Sir,
Disseminated co-infection with herpes simplex virus (HSV) and varicella zoster virus (VZV) has been reported in immunocompromised patients,[1],[2] and only localised dermatomal herpes simplex and varicella zoster viruses co-infection has been rarely seen in immunocompetent patients.[3],[4] We hereby present a case of both viruses causing disseminated infection in an immunocompetent patient. A 64-year-old Chinese gentleman, with a past medical history of a right temporo-occipital intracranial arteriovenous malformation (AVM) resulting in epilepsy and organic brain syndrome, was admitted to Neurosurgery ward for a breakthrough seizure secondary to intraventricular hemorrhage. He underwent uneventful insertion of an emergent temporary right frontal external ventricular drain on the same day, and subsequent replacement with a left ventriculo-peritoneal shunt 15 days later. He was stable after the surgery and his Glasgow Coma Scale (GCS) was 14. On postoperative day 4, he developed a fever which reached 40.6°C the next day, coinciding with the appearance of a widespread blistering rash.
On physical examination, the patient was febrile, tachycardic, hypotensive, and his GCS was 8. There were multiple, tense, clear, fluid-filled small bullae on his trunk, back, upper limbs [Figure - 1], and macerated erosions on the right axilla and periumbilical area. There were extensive erosions with crusts over his cheeks and forehead [Figure - 2], and weepy erosions on the glans penis and shaft. Laboratory investigations revealed leukocytosis of 16.0 × 10(9) /L, normal hemoglobin and platelet levels. There was mild transaminitis with elevation of alanine transaminase from 49 to 179 U/L and aspartate transaminases from 46 to 149 U/L. Renal function tests were normal.
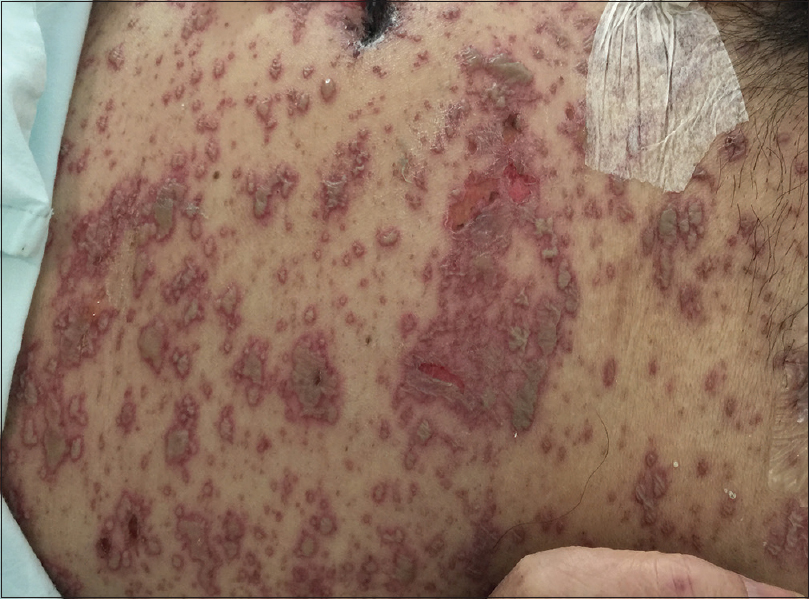 |
| Figure 1a: Widespread multiple tense clear fluid-filled small bullae on the trunk. The patient was hemodynamically unstable and required continuous cardiac monitoring |
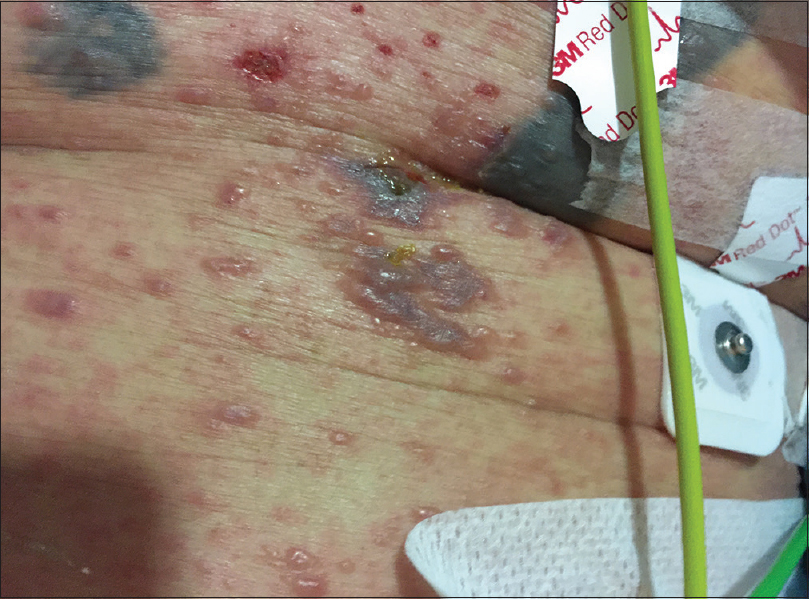 |
| Figure 1b: Close up of bullae on an erythematous base on the chest |
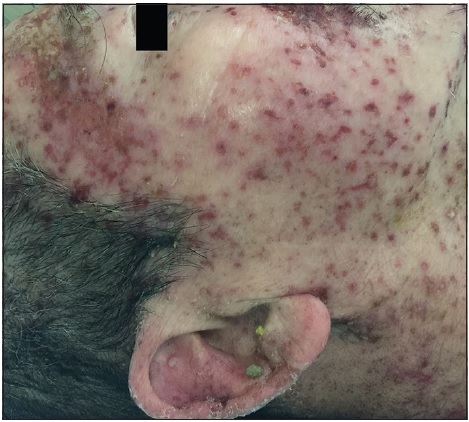 |
| Figure 2a: Extensive crusted erosions over the forehead |
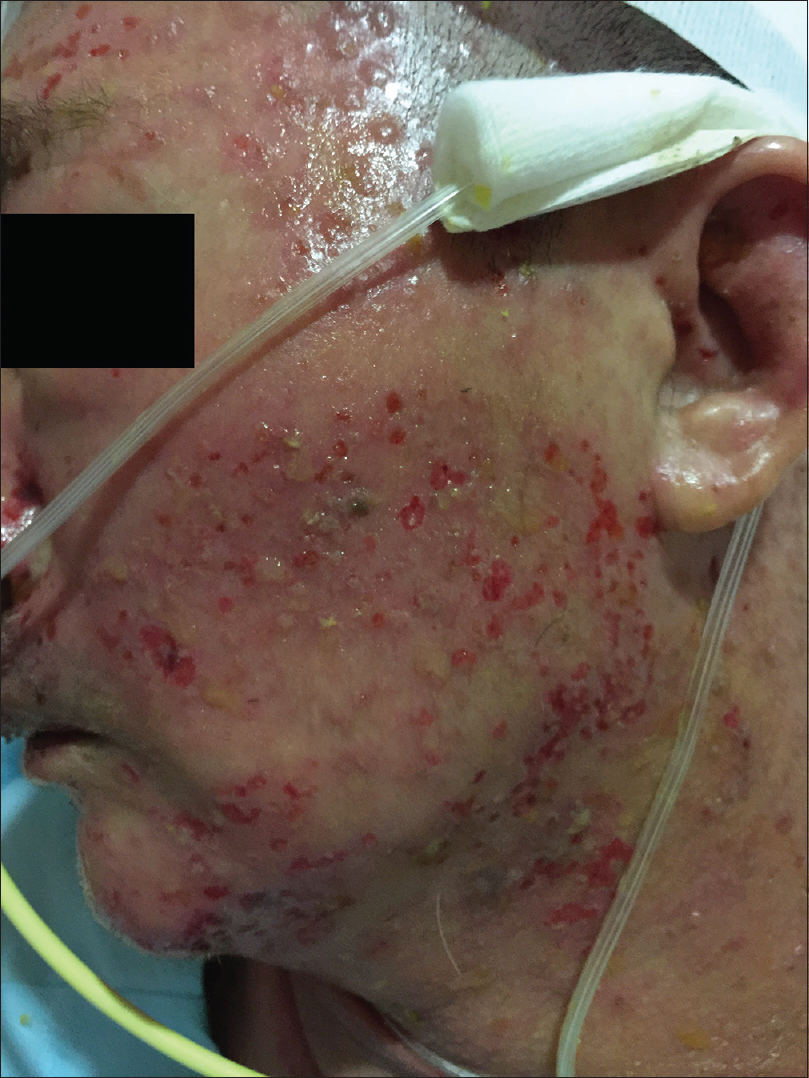 |
| Figure 2b: Extensive crusted erosions over the cheeks |
Upon consultation with a dermatologist and infectious disease physician on day 2 of the onset of rash, the clinical diagnosis was disseminated herpes simplex versus varicella zoster virus infection and investigations were performed. Serum real-time quantitative polymerase chain reaction (PCR) for HSV Type 1 (LightCycler, Roche Diagnostics) was positive at 4.8E+03 copies of viral DNA/mL. Herpes simplex virus PCR from a blister swab was negative. Serum VZV IgM and IgG antibodies enzyme linked immunosorbent assay (Euroimmun, Germany) and a blister swab for varicella zoster virus immunofluorescence returned positive. Tzanck smear was not performed. Blood aerobic and anaerobic cultures were negative for bacterial growth. Chest radiograph revealed interval development of mild air space opacification in the right lower zone, suggestive of an infection.
The patient had been commenced on empirical intravenous acyclovir (10 mg/kg body weight 8 hourly) at day 3 of disease. He was also given meropenem and vancomycin to cover for healthcare-associated pneumonia. He responded well with hemodynamic stabilisation over the next few days and progressive crusting and resolution of the vesicular rash over the next 2 weeks.
We were unable to find any previous reports of both viruses resulting in a disseminated infection in an immunocompetent host. We looked for possible causes of immunosuppression, but while this patient had a history of epilepsy and organic brain syndrome related to an arteriovenous malformation, he had no other known pre-existing conditions that could have contributed to a lowered immunocompetent state. His usual medications of sodium valproate and olanzapine did not include any immunosuppresants. In particular, there was no use of steroid administered for cerebral edema or any other reason. Screening tests for underlying immunocompromised states such as retroviral infection and diabetes mellitus also returned negative. On the other hand, as the systemic infection developed in the postoperative period, it is possible that stress associated with the surgery with consequent cortisol release contributed to transient lowered immunity. A rare possibility is that of uncommon genetic variants such as TLR3[5] or UNC-93B deficiency,[6] resulting in impaired anti-viral immune responses that have been linked to the cause of HSV-1 encephalitis.
A differential diagnosis of Kaposi varicelliform eruption was also considered but there was no preceding skin pathology such as eczema. In addition, we considered the likelihood of false positive investigation results due to cross-reactivity of shared epitopes on the proteins expressed by both viruses of the same herpesvirinae subfamily. However, the specificity of the LightCycler-PCR for detecting herpes simplex virus had been previously confirmed by testing it against varicella zoster virus DNA, with no cross-reactivity demonstrated.[7] The zoster IgG and IgM assays test kits also stated no cross-reactivity with herpes simplex virus.
In summary, we report a case of disseminated herpes simplex and varicella zoster co-infection in an immunocompetent host and wish to raise physicians' awareness of such an occurrence even in immunocompetent patients especially in the postoperative period.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patients understood that his names and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Curley MJ, Hussein SA, Hassoun PM. Disseminated herpes simplex virus and varicella zoster virus coinfection in a patient taking thalidomide for relapsed multiple myeloma. J Clin Microbiol 2002;40:2302-4.
[Google Scholar]
|
| 2. |
Krishnaram AS, Sachan Y, Rani GG. Co-reactivation of varicella zoster virus and herpes simplex virus with disseminated cutaneous lesions in a lymphoma patient. Indian J Dermatol Venereol Leprol 2013;79:709-11.
[Google Scholar]
|
| 3. |
Giehl KA, Müller-Sander E, Rottenkolber M, Degitz K, Volkenandt M, Berking C, et al. Identification and characterization of 20 immunocompetent patients with simultaneous varicella zoster and herpes simplex virus infection. J Eur Acad Dermatol Venereol 2008;22:722-8.
[Google Scholar]
|
| 4. |
Park HH, Lee MH. Concurrent reactivation of varicella zoster virus and herpes simplex virus in an immunocompetent child. J Korean Med Sci 2004;19:598-600.
[Google Scholar]
|
| 5. |
Guo Y, Audry M, Ciancanelli M, Alsina L, Azevedo J, Herman M, et al. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J Exp Med 2011;208:2083-98.
[Google Scholar]
|
| 6. |
Casrouge A, Zhang SY, Eidenschenk C, Jouanguy E, Puel A, Yang K, et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science 2006;314:308-12.
[Google Scholar]
|
| 7. |
Burrows J, Nitsche A, Bayly B, Walker E, Higgins G, Kok T, et al. Detection and subtyping of Herpes simplex virus in clinical samples by LightCycler PCR, enzyme immunoassay and cell culture. BMC Microbiol 2002;2:12.
[Google Scholar]
|
Fulltext Views
5,368
PDF downloads
2,043





