Translate this page into:
Does rapamycin induce melanin formation? An in vitro study assessing the effect of rapamycin on normal cultured melanocytes
Correspondence Address:
Davinder Parsad
Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh
India
| How to cite this article: Kumaran MS, Srivastava N, Vinay K, Bhardwaj S, Parsad D. Does rapamycin induce melanin formation? An in vitro study assessing the effect of rapamycin on normal cultured melanocytes. Indian J Dermatol Venereol Leprol 2019;85:330-332 |
Sir,
Rapamycin, the mammalian target of rapamycin pathway inhibitor, has been extensively used in organ transplantation and oncology.[1] The activation of the mammalian target of rapamycin pathway, caused by loss of function mutation in tuberous sclerosis complex gene, has been shown to cause autophagic deficiency in melanocytes leading to reduced pigmentation.[2] Treatment with rapamycin reverses the autophagic dysfunction and causes repigmentation of ashleaf spots in patients with tuberous sclerosis.[2],[3] Rapamycin-induced repigmentation in tuberous sclerosis complex has also been attributed to reduced endoplasmic reticulum and mitochondrial oxidative stress levels.[4] The currentin vitro study was performed to assess the effects of rapamycin on normal melanocytes with regard to melanin formation, melanocyte maturation and E-cadherin expression.
Melanocyte cell cultures were established from skin grafts harvested from normal skin of five melasma patients attending the pigmentary clinic of the Postgraduate Institute of Medical Education and Research, Chandigarh, India as per the standard protocol (PromoCell C-24010).[5] Melanocytes were used in the density of 3 × 10[4] cells/cm[2]. Further, these cultures were treated with different concentrations (2, 100 and 150 nM) [Figure - 1] of rapamycin and thereafter, cell proliferation (MTT assay and Ki-67), viability (trypan blue staining), melanin and tyrosinase content and expression of adhesion molecule E-cadherin were assessed.[5] All experiments were performed five times in sets of triplicate for each concentration of rapamycin as well as the control. The Ethics Committee of the institute approved the study and the recruited patients signed an informed consent form (NK/902/RES/130).
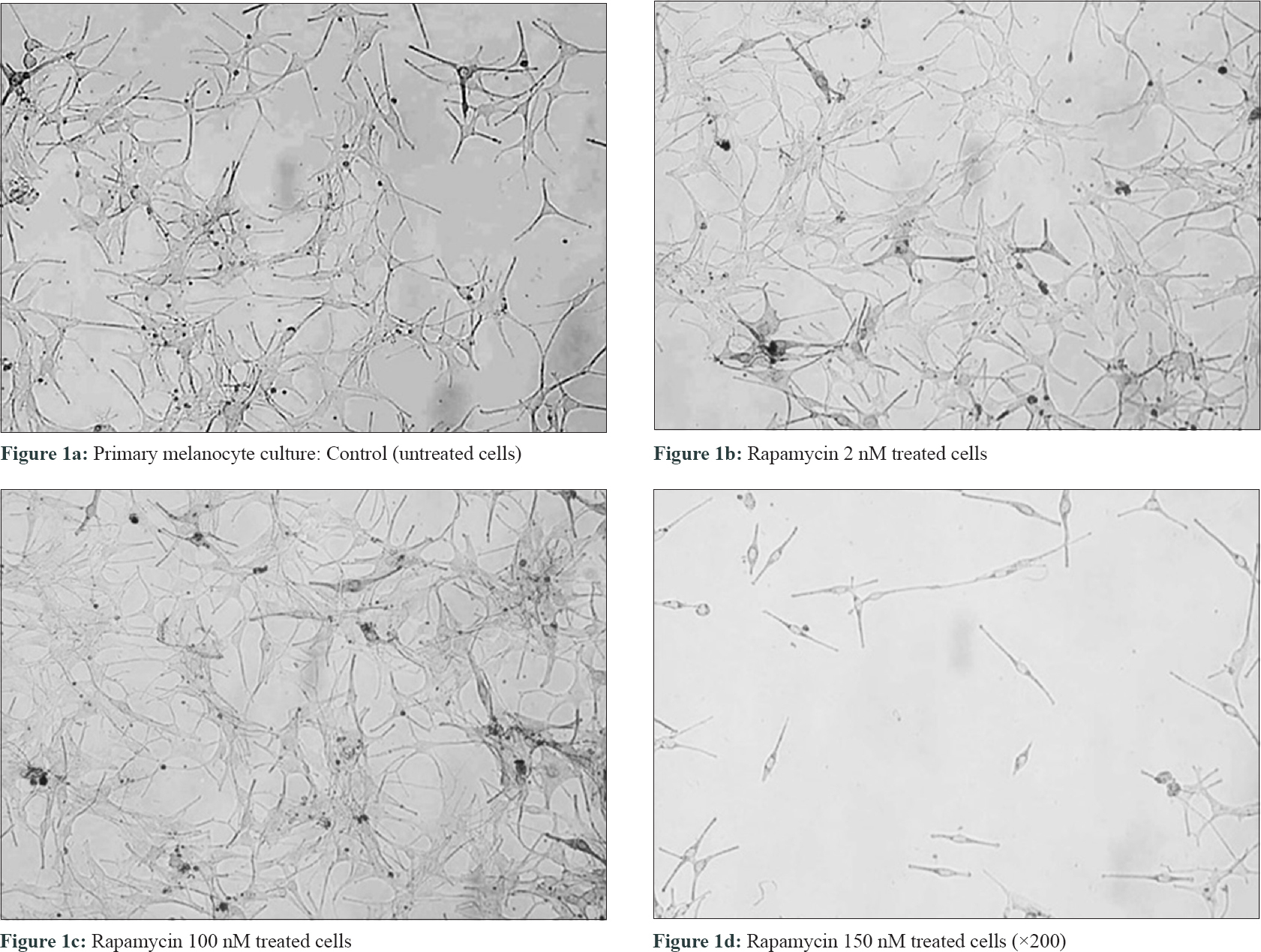 |
| Figure 1: |
On cell proliferation analysis of melanocytes by MTT assay at 100 nM rapamycin concentration, cell proliferation was found to be increased [5.5 ± 0.4 vs. 5.0 ± 0.1 ng/ml, P = 0.05, [Figure - 1] and [Figure - 2]a, and at 150 nM concentration, it was decreased (4.7 ± 0.2 vs. 5.0 ± 0.1 ng/ml, P = 0.19). Tyrosinase level also significantly increased at 2 nM (13.5 ± 0.05 vs. 12.8 ± 0.3 ng/ml, P = 0.001) and 100 nM concentrations (15.4 ± 0.5 vs. 12.8 ± 0.3 ng/ml, P = 0.001). However, at 150 nM concentration (8.7 ± 0.03 vs. 12.8 ± 0.3 ng/ml, P = 0.001), tyrosinase level had significantly decreased [Figure - 2]b. Melanin production [Figure - 2]c and E-cadherin expression were not affected after adding rapamycin. Ki-67 expression was not found on cultured melanocytes before as well as after treatment with rapamycin.
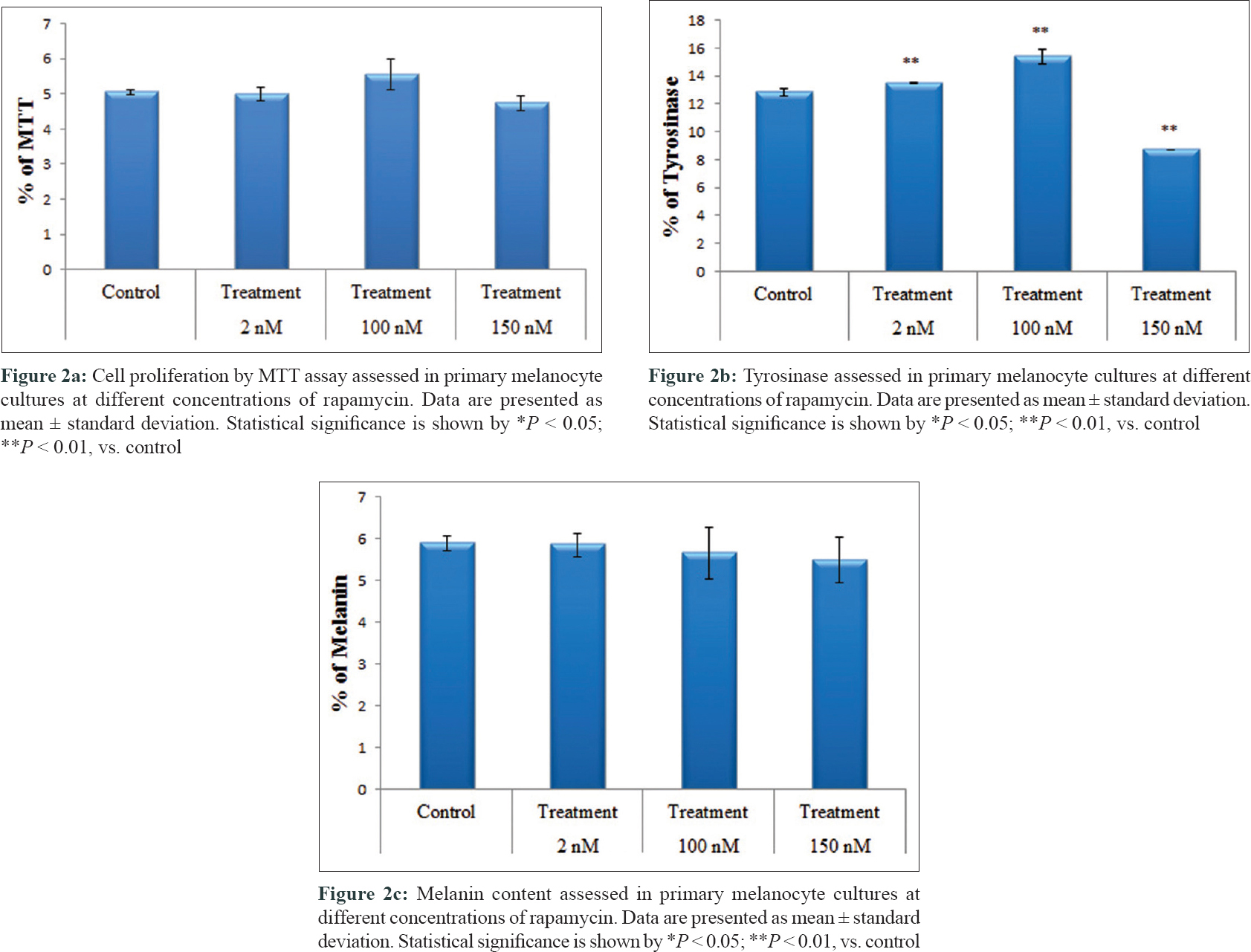 |
| Figure 2: |
Mammalian target of rapamycin, a serine-threonine kinase, is associated with a number of cellular processes such as cell growth, proliferation, cell motility, cellular metabolism and autophagy. Recently, studies have demonstrated that specific inhibitors of phosphoinositide 3-kinase, such as wortmannin and LY294002, stimulate melanin production in mouse and human melanoma cells, proposing that phosphoinositide 3-kinase pathway might be involved in the regulation of melanogenesis.[6] Studies assessing the effect of rapamycin on melanoma cell cultures have observed an increase in melanogenesis in melanoma cell culture, which was demonstrated by an increase in baseline tyrosine levels, using tyrosinase assay method.[7] The changes were maximum at 100 nM concentration of the drug, which corroborates with our observations. Although in our study we noted an increase in tyrosine levels by tyrosinase assay method, we did not observe an associated increase in melanization using melanin content assay method, which was contrary to the findings by Hah et al.[7] The reason for the same can be the alteration in intermediate factors involved in melanin formation from tyrosine. Among various concentrations of rapamycin, uniquely at 100 nM concentration, an increase in the number of melanocytes proliferation was noted, which was not observed by Hah et al.[7] The significance of this finding in this study needs to be explored. Anti Ki-67 antibody assessment showed that this proliferation has no malignant property.
This study demonstrates that rapamycin has a peculiar effect on melanocytes, its effect being dose-dependent. This suggests that mammalian target of rapamycin pathway inhibitors can be explored further as a treatment option in a socially stigmatized disorder such as vitiligo. Although most of our findings are preliminary, future studies can supplement our findings.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Saunders RN, Metcalfe MS, Nicholson ML. Rapamycin in transplantation: A review of the evidence. Kidney Int 2001;59:3-16.
[Google Scholar]
|
| 2. |
Yang F, Yang L, Wataya-Kaneda M, Hasegawa J, Yoshimori T, Tanemura A, et al. Dysregulation of autophagy in melanocytes contributes to hypopigmented macules in tuberous sclerosis complex. J Dermatol Sci 2018;89:155-64.
[Google Scholar]
|
| 3. |
Wataya-Kaneda M, Tanaka M, Yang L, Yang F, Tsuruta D, Nakamura A, et al. Clinical and histologic analysis of the efficacy of topical rapamycin therapy against hypomelanotic macules in tuberous sclerosis complex. JAMA Dermatol 2015;151:722-30.
[Google Scholar]
|
| 4. |
Yang F, Yang L, Wataya-Kaneda M, Yoshimura T, Tanemura A, Katayama I, et al. Uncoupling of ER/Mitochondrial oxidative stress in mTORC1 hyperactivation-associated skin hypopigmentation. J Invest Dermatol 2018;138:669-78.
[Google Scholar]
|
| 5. |
Kumar R, Parsad D, Kanwar A, Kaul D. Development of melanocye-keratinocyte co-culture model for controls and vitiligo to assess regulators of pigmentation and melanocytes. Indian J Dermatol Venereol Leprol 2012;78:599-604.
[Google Scholar]
|
| 6. |
Bertolotto C, Abbe P, Hemesath TJ, Bille K, Fisher DE, Ortonne JP, et al. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J Cell Biol 1998;142:827-35.
[Google Scholar]
|
| 7. |
Hah YS, Cho HY, Lim TY, Park DH, Kim HM, Yoon J, et al. Induction of melanogenesis by rapamycin in human MNT-1 melanoma cells. Ann Dermatol 2012;24:151-7.
[Google Scholar]
|
Fulltext Views
3,399
PDF downloads
1,262





