Translate this page into:
Drug reaction with eosinophilia and systemic symptoms: Observations from a tertiary care institution
Correspondence Address:
Sarita Sasidharanpillai
'Rohini', Girish Nagar, Nallalom PO, Kozhikode - 673 027, Kerala
India
| How to cite this article: Sasidharanpillai S, Riyaz N, Rajan U, Binitha MP, Khader A, Reena Mariyath OK, John R, Puravoor J. Drug reaction with eosinophilia and systemic symptoms: Observations from a tertiary care institution. Indian J Dermatol Venereol Leprol 2014;80:221-228 |
Abstract
Background: Drug reaction with eosinophilia and systemic symptoms (DRESS) is a severe drug reaction which can mimic a viral infection, an autoimmune disease or a neoplastic disease. Aims: To study the clinical and epidemiological aspects of DRESS and to identify the precipitating drugs. Methods: All patients admitted to the dermatology ward of our tertiary care hospital from 1 st October 2010 to 30 th September 2013 with probable or definite DRESS as per the RegiSCAR scoring system were included in this prospective study. The clinical manifestations observed in the study population were studied and the common offending drugs were identified. Results: During the 3 year study period, 26 patients fulfilled criteria for probable or definite DRESS. In more than 50% of cases, the culprit drug was phenytoin. Most common symptoms observed were fever, rash and facial edema. Liver was the most common internal organ affected. Most of the patients responded to withdrawal of the drug and administration of steroids for 3-6 weeks. One patient with dapsone-induced DRESS died. Conclusions: Intense facial erythema and edema and an elevated eosinophil count were not found to be bad prognostic factors. In most instances the flare ups during the course of the disease could be managed with a slower tapering of steroids. More prospective studies on DRESS are required to assess the prognostic factors and to formulate better diagnostic criteria.INTRODUCTION
Chaiken et al., in 1950, reported a patient who had developed rash associated with lymphadenopathy and multiorgan failure long after introducing phenytoin. [1] Since then several cases have been reported with similar reactions to many other drugs. Later the term ′drug reaction with eosinophilia and systemic symptoms (DRESS)′ was coined to describe this disorder, [2] but this was questioned by many as eosinophilia was not evident in all patients. A Japanese consensus group proposed that the term "drug-induced hypersensitivity syndrome (DIHS)" be used instead of DRESS and suggested a new diagnostic criteria. [3]
It is currently believed that DIHS represents the most severe cases of DRESS and is associated with reactivation of human herpesvirus 6 (HHV-6) in a large majority of patients while this is seen only in a limited number of patients with DRESS. [4]
Several authorities proposed different definitions for DIHS or DRESS, [5] but no consensus has been reached so far probably due to the highly variable and not so distinctive clinical features.
′The RegiSCAR study group provided a broad diagnostic criteria that has high sensitivity, but lacks specificity.Kardaun et al. put forth a scoring system (RegiSCAR DRESS validation score) to classify drug reactions as definite, probable, possible and not DRESS. [6]
Many aspects of the pathogenesis and disease progression in DRESS still remain unknown. The reaction pattern may vary in different populations, based on the genetic characteristics and the commonly prescribed drugs.
We conducted a prospective study on the clinical patterns and the precipitating drugs among patients who attended our tertiary care institution with definite or probable DRESS.
METHODS
Ethical clearance was obtained from the institutional ethics committee and written informed consent was taken from all study subjects. Patients who were admitted in the dermatology department of our institution from October 1, 2010 to September 30, 2013 and who satisfied the criteria for probable adverse drug reaction on World Health Organization causalty assessment were evaluated. Rechallenge with the suspected drug was not carried out.
A pre-designed proforma was used to collect data regarding age, sex, precipitating drug, underlying condition for which the offending drug was introduced, latent interval between the drug intake and the onset of symptoms, evolution of symptoms and past medical and drug history including past drug allergies.
Each patient was carefully examined with respect to the type and distribution of rash, presence or absence of facial erythema, facial and pedal edema, mucosal lesions, lymphadenopathy, and systemic involvement. Complete hemogram, renal and liver function tests and absolute eosinophil count were performed at the time of admission. Liver function tests and absolute eosinophil count, if found within normal limits, were repeated at an interval of five days till the day of discharge. Peripheral smear analysis for malarial parasites and atypical cells, ultrasound examination of abdomen and pelvis, electrocardiogram, blood culture and serology for human immunodeficiency virus (HIV) infection were carried out in each patient. Antinuclear antibody profile, chest radiography and serology for infectious mononucleosis, leptospirosis, typhoid fever, rickettsia, dengue, chikungunya and hepatitis B, C and A infections were performed where relevant. Skin biopsy was carried out only in doubtful cases.
Lymphadenopathy, rash suggestive of DRESS, eosinophilia and internal organ involvement were diagnosed on the basis of the RegiSCAR study group definitions. [7] Atypical lymphocytosis was considered if the atypical lymphocyte count was more than 5% in the peripheral smear.
Response to withdrawal of the suspected drug and the treatment received were carefully documented. Flare ups experienced during the course of the disease were recorded and the patients were followed up till they completed treatment for the adverse drug reaction.
Patients who satisfied the criteria for definite or probable DRESS as per the RegiSCAR scoring system put forth by Kardaun et al. [6] were studied with respect to the epidemiology, the reaction patterns as well as the drugs precipitating the adverse reaction. Culprit drugs were determined based on the chronology from the introduction of the drug to the onset of symptoms and any known association between the suspected drug and DRESS.
RESULTS
During the 3 year study period we made a diagnosis of probable adverse drug reaction in 129 patients. Of these, 26 patients belonged to the category of probable (11) or definite (15) DRESS [Table - 1]. Fourteen were females. Eight drugs were identified as producing DRESS [Table - 1]. In one instance where the patient started taking phenytoin and phenobarbitone simultaneously, we could not determine the exact causative drug.
The age of affected patients ranged from 3 to 65 years with a mean of 37.3 years. In 14 (53.8%) out of the 26 cases, the culprit drug was phenytoin.
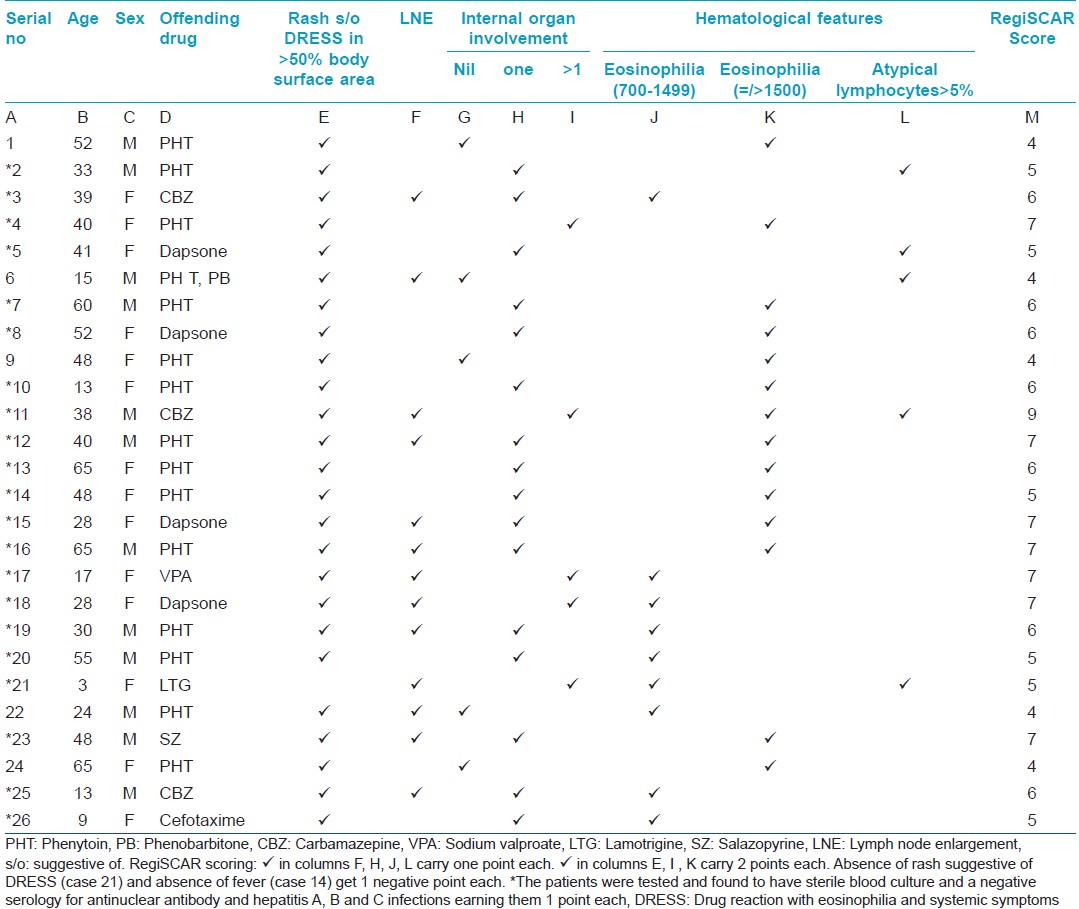
The latent period between drug intake and the onset of symptoms varied from 7 to 90 days with an average of 27.2 days. The average latent period between drug intake and the appearance of symptoms was longer in carbamazepine-induced DRESS [Figure - 1].
 |
| Figure 1: Average latent period between the drug intake and the onset of symptoms in DRESS |
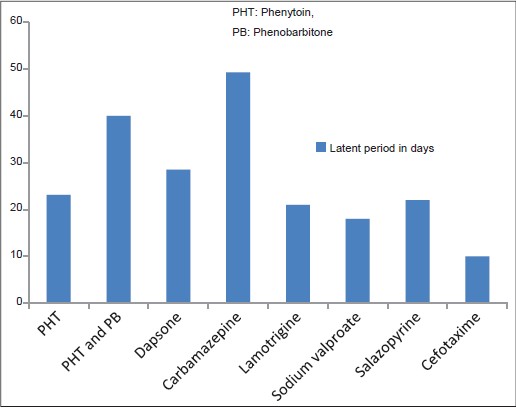
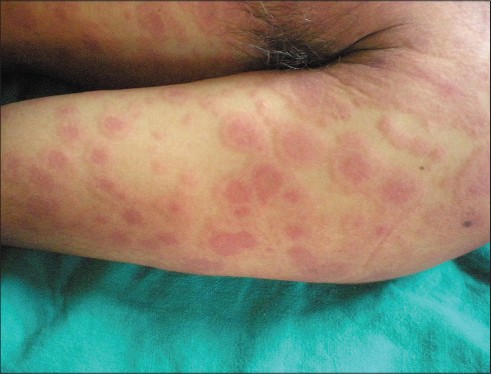
The most common symptoms observed were fever, rash [Table - 2] and facial edema. The initial symptoms were fever and rash in 15 (57.7%) cases. Rash succeeded the fever in nine patients.
Facial edema was observed in 25 (96.2%) of 26 patients [Figure - 2].
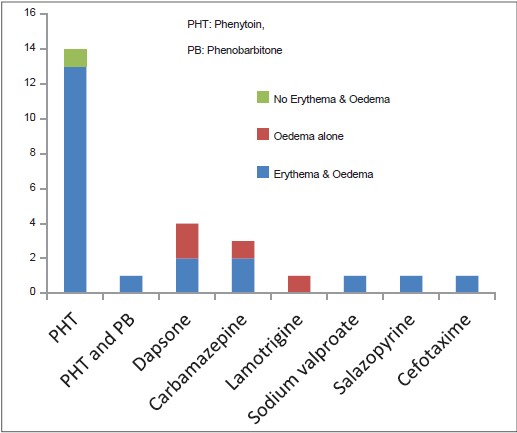 |
| Figure 2: Erythema and edema of face in DRESS |
Facial erythema that subsided with scaling was present in 21 (80.8%) out of 26 patients [Figure - 2]. Facial erythema and edema were more prominent in men than in women. Phenytoin was associated with severe edema and intense erythema of the face though it was also the causative drug in the lone patient who developed neither edema nor erythema of the face.
Twenty (76.9%) patients manifested a maculopapular rash. Three of them developed pustules scattered over the face, back and extremities [Figure - 3]. The offending drug was phenytoin in two and dapsone in one. The initial maculopapular rash progressed to exfoliative dermatitis in two patients and the offending drugs were carbamazepine and salazopyrine. The initial rash was exfoliative dermatitis in four patients (caused by dapsone in 2, cefotaxin in 1, phenytoin in 1 patient) and erythema multiforme in another (caused by dapsone) [Figure - 4]. The rash involved the entire body in 20 patients and spared the forearms, legs, palms and soles of 5 patients.
 |
| Figure 3: Pustules in DRESS |
 |
| Figure 4: Erythema multiforme in DRESS |
Mucosae were spared in 7 (26.9%) cases. In 11 (42.4%) patients, mucosal involvement was limited to dryness and scaling of lips. Conjunctival (eight patients), oral (two patients) and genital (two patients) mucosae were affected occasionally.
Lymphadenopathy was detected in 50% of patients for which no other cause could be identified.
Elevation of absolute eosinophil count was observed in nine (34.6%) patients each during the first and second week of disease onset, during the third week in three (11.5%) patients and later than that in two (7.7%) others. It was within normal limits throughout the course of disease in three (11.5%) patients [Table - 1].
Atypical lymphocytosis was observed in the peripheral smear of five (19.2%) patients.
Deranged liver function test was noted during the first week of disease in nine (34.6%), in the second week in seven (26.9%), in the third week in two (7.7%) and later than that in three (11.5%) patients.
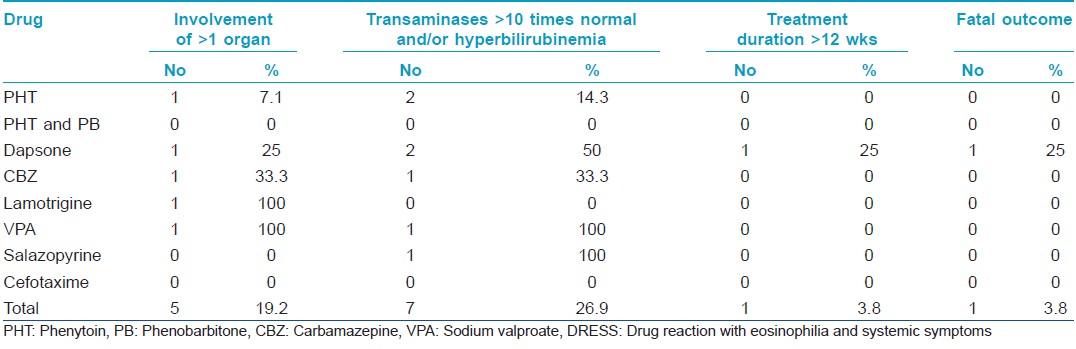
Hyperbilirubinemia (conjugated or unconjugated) or more than ten-fold elevation of liver transaminases were observed in three males and four females [Table - 3]. No hepatic abnormality was noted in three males and two females in all of who the precipitating drug was phenytoin (one of them was also receiving phenobarbitone).
Organomegaly was detected in six (23.1%) patients, 1 man and 5 women. Hepatomegaly was associated with dapsone intake in 2 patients, splenomegaly with carbamezapine intake in 1 patient and hepatosplenomegaly with dapsone, sodium valproate and lamotrigine in 1 patient each.
Other systems found affected in the study group were the pulmonary and renal systems. Three patients, 2 women and 1 man, developed pneumonitis associated with the intake of sodium valproate, phenytoin and carbamazepine in 1 patient each. Two women developed nephritis and acute renal failure after lamotrigine and dapsone, respectively.
Prednisolone 1 mg/kg or an equivalent dose of dexamethasone was given to those with severe DRESS (more than 10-fold elevation of transaminases or hyperbilirubinemia or those having involvement of more than one organ system) [Table - 3]. The rest received prednisolone or prednisolone equivalent at a dose of 0.5 mg/kg body weight. Steroids were tapered every 5-7 days.
Twenty-three (88.5%) of the 26 patients needed treatment for 21-42 days. Three patients who developed disease flares manifested as exacerbation of rash and liver function derangement when steroids were tapered to 20 mg prednisolone/equivalent, responded to an increase in dose followed by a slower withdrawal. Phenytoin, carbamazepine and dapsone were the offending drugs in these patients. The two patients with DRESS induced by dapsone and carbamazepine developed recurrent flare ups. The former needed treatment for 6 months and the latter for three months.
One patient died (case no. 5 in [Table - 1]). This patient, who developed DRESS after dapsone [Figure - 5]a, had hyperbilirubinemia, elevated liver transaminases, 23% atypical lymphocytes in peripheral smear and a normal absolute eosinophil count. Dapsone was withdrawn and dexamethasone 8 mg intravenously once daily was started. She improved and was discharged on the 19 th hospital day and advised to continue prednisolone, 30 mg daily. She stopped taking steroids on her own three days after the discharge and was readmitted 14 days later with toxic epidermal necrolysis [Figure - 5]b. Intravenous dexamethasone and immunoglobulin G could not arrest the disease progression; she developed acute renal failure and expired on the fifth hospital day.
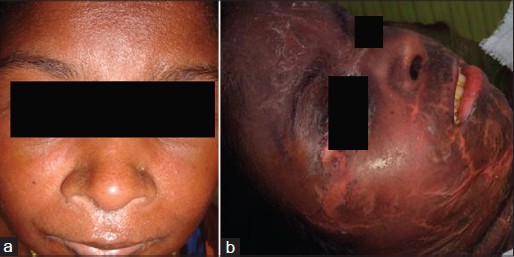 |
| Figure 5: (a) Erythema and edema of the face in a patient with dapsone induced DRESS. (b) The same patient when she progressed to toxic epidermal necrolysis |
DISCUSSION
There was a slight preponderance of women in our study, as noted in previous reports. [5] The mean age of our patients was comparable to the data published by Cacoub et al., but was lower than that reported in certain other studies. [5],[8]
The latent period between drug intake and the onset of symptoms was similar to that previously reported [9],[10] including the longer latent period for carbamazepine-induced DRESS.
The different types of rashes noted in our patients have been previously described in DRESS. [4],[5],[8] as was our finding of a maculopapular rash being the most common type of eruption. [5],[8] All the eruptions in our patients were suggestive of DRESS. The three patients who developed pustular lesions in addition to the maculopapular rash had to be distinguished from acute generalized exanthematous pustulosis (AGEP). The latent period between the drug intake and onset of symptoms is of short duration in AGEP. AGEP starts with diffuse erythema followed soon by the appearance of disseminated non-follicular pustules with a flexural predilection. Compared to AGEP, pustules (when present) in DRESS are follicular, lack flexural accentuation and are mainly limited to the face and upper thorax, though one of our patients had pustules affecting the extremities. Withdrawal of the causative drug leads to a quick resolution in AGEP, whereas slow recovery is the rule in DRESS. [11] Aromatic antiepileptic drugs like phenytoin and dapsone, which were the culprit drugs in the patients with pustules in our study, are common offenders in DRESS but are not usually implicated in AGEP. Likewise macrolides, quinolones and aminopenicillins, important triggers for AGEP, are not clearly associated with DRESS.
DRESS without rash has been reported earlier and was observed in one of our patients (case no. 21 in [Table - 1]). [8] The child developed high grade fever, facial and pedal edema, generalized lymphadenopathy, liver function derangement with hepatosplenomegaly, nephritis and hematological changes 3 weeks after starting lamotrigine. Autoimmune, infective and neoplastic causes were ruled out by meticulous clinical and laboratory work up and our diagnosis of DRESS to lamotrigine was further confirmed by her response to withdrawal of lamotrigine and administration of systemic steroids.
Facial edema (96.2%) and facial erythema (80.8%) documented in our patients were higher than the observations in some studies, but others documented comparable results. [5],[9],[12]
Unlike previous reports [9] facial edema was not associated with an increased risk of internal organ involvement in our study. The two patients (one male and one female) who presented with the most severe facial edema in our study [Figure - 6] had no internal organ involvement other than lymphadenopathy in one patient. The female patient had the highest eosinophil count detected in the study group (14,000 cells/mm 3 ), whereas the male showed a normal eosinophil count. Both cases were associated with phenytoin intake.
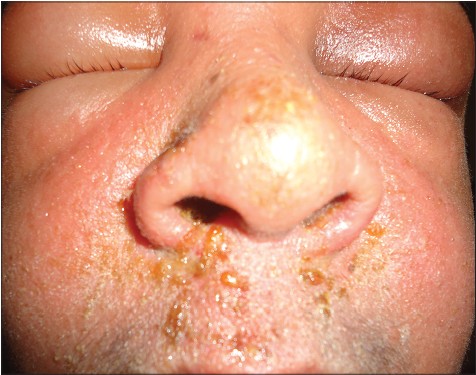 |
| Figure 6: Intense erythema and edema of the face in phenytoin induced DRESS |
Others noted fever as frequently as in our study (96.2%) [9] though a lower frequency has also been reported. [5],[8] Our finding of lympadenopathy in 50% of patients fell between the 18% and 75% reported previously. [12] We found mucosal involvement in 73.1% of our patients, higher than in previous reports (48%). [8],[12]
Hepatic involvement was the most common systemic abnormality detected. [8],[12] It has been suggested that a delay in withdrawal of the suspected drug may increase the risk of hepatic involvement with jaundice. [9] However, we found that this complication depended on the causative medication rather than the delay in drug withdrawal. One of the dapsone-induced DRESS patients sought medical advice on the second day of illness, was diagnosed and treated promptly, yet had jaundice while in two instances phenytoin-induced DRESS was not associated with this complication in spite of a treatment delay of 30 days.
Five (71.4%) out of the seven cases with a more than 10 fold elevation of transaminases or hyperbilirubinemia were caused by drugs other than phenytoin, though the latter was the causative drug in more than 50% of the study population. A relatively less severe DRESS following phenytoin was also observed by others. [12]
Only 3 (21.4%) of the 14 patients who had an eosinophil count above 1500 were noted to have severe manifestations. A higher absolute eosinophil count was not indicative of severe DRESS in our patients. Certain studies were in concordance with our observation while others reported severe disease in those with marked eosinophilia. [12]
On the contrary, three (60%) out of the five patients who showed atypical lymphocytosis in the peripheral smear had severe disease with fatal outcome in one. The lower percentage of patients manifesting atypical lymphocytosis in our study could be attributed to the 5% cut off mark adopted by us, whereas according to the RegiSCAR scoring system the presence of atypical lymphocytes in the peripheral smear was enough to get one point.
In six patients the offending drug was withdrawn even before a diagnosis of drug reaction was made because liver function derangement was detected. In four of the six patients, rash and facial edema showed improvement after stopping the drug. In one patient the rash resolved only to reappear after 6 days. The rash and facial oedema in the sixth patient showed improvement only after starting steroids. The liver transaminases in one patient and absolute eosinophil count in two patients continued to rise even after withdrawal of the suspected drug till systemic steroids were administered. The rest showed a decline in both, but normal levels were reached only after steroids were started. In all six patients, high grade fever persisted till the administration of steroids.
The mortality rate of 3.8% in our study was much lower than in previous reports (10-40%). [9] This could be attributed to the absence of allopurinol or minocycline as causative drugs in our patient group as these two drugs are known to induce life-threatening DRESS. [5],[9],[12]
The only death in the study group was precipitated by the premature withdrawal of systemic steroids. The progression of the recrudescent eruption to fatal toxic epidermal necrolysis in this patient after recovery from the initial DRESS was a significant observation but it was not clear why the disease evolved in this manner.
Seven (70%) of the 10 patients who had severe disease were females. A female preponderance among severe DRESS cases was noted in previous studies as well. [13]
Dapsone-induced DRESS showed certain features such as a fewer number of patients manifesting maculopapular rash, (25% vs 76.9% of the total), facial erythema in only 50% of cases and severe manifestations in 75% of cases. Whether the fact that all dapsone-induced patients were females had contributed to the disease severity needs further evaluation.
As the study was limited to patients who required inpatient care we might have missed milder forms of DRESS. The other major limitation of this study was our inability to assess the role of HHV-6 in all cases. Of the four patients assessed, one showed evidence of HHV-6 reactivation. [14],[15] Another major drawback of this study was our failure to follow up the patients to determine the delayed autoimmune complications of DRESS.
SUMMARY
DRESS is not an uncommon disease and has to be considered in the differential diagnosis of fever with or without rash and systemic involvement in any patient, who has started taking drugs known to produce this adverse reaction during the past 3-4 months. The absence of constitutional symptoms, rash or eosinophilia by themselves does not rule out DRESS. Association of phenytoin with less severe and of dapsone, female sex and atypical lymphocytosis with more severe DRESS needs further evaluation. Withdrawal of the offending drug and administration of prednisolone achieved cure in more than 95% of cases. In most instances, flare ups during prednisolone withdrawal could be managed with an increase in steroid dose followed by slower tapering. Abrupt and premature steroid withdrawal proved fatal in 1 patient who stopped treatment on her own. Further prospective studies may enable us to formulate better diagnostic criteria as well as improve management of DRESS.
ACKNOWLEDGMENT
We are grateful to all the faculty and postgraduates in the department for their invaluable help in conducting this study.
| 1. |
Chaiken BH, Goldberg BI, Segal JP. Dilantin sensitivity; report of a case of hepatitis with jaundice, pyrexia and exfoliative dermatitis. N Engl J Med 1950;242:897-8.
[Google Scholar]
|
| 2. |
Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin Cutan Med Surg 1996;15:250-7.
[Google Scholar]
|
| 3. |
Shiohara T, Iijima M, Ikezawa Z, Hashimoto K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivation. Br J Dermatol 2007;156:1083-4.
[Google Scholar]
|
| 4. |
Criado PR, Avancini J, Santi CG, Medrado AT, Rodrigues CE, de Carvalho JF. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): A complex interaction of drug, viruses and the immune system. Isr Med Assoc J 2012;14:577-82.
[Google Scholar]
|
| 5. |
Ang CC, Wang YS, Yousuf EM, Tay YK. Retrospective analysis of drug induced hypersensitivity syndrome: A study of 27 patients. J Am Acad Dermatol 2010;63:219-27.
[Google Scholar]
|
| 6. |
Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: Does a DRESS syndrome really exist? Br J Dermatol 2007;56:609-11.
[Google Scholar]
|
| 7. |
Chen YC, Chang CY, Cho YT, Chiu HC, Chu CY. Reply to: ''Using a diagnostic score when reporting the long-term sequelae of the drug reaction with eosinophilia and systemic symptoms'' J Am Acad Dermatol 2013;69:1060-2.
[Google Scholar]
|
| 8. |
Cacoub P, Musette P, Descamps V, Meyer O, Spiers C, Finzi L, et al. The DRESS Syndrome: A Literature Review. Am J Med; 2011:588-597.
[Google Scholar]
|
| 9. |
Kumari R, Timshine DK, Thappa DM. Drug Hypersensitivity Syndrome. Indian J Dermatol Venereol Leprol 2011;77:7-15.
[Google Scholar]
|
| 10. |
Gentile I, Talamo M, Borgia G. Is the drug-induced hypersensitivity syndrome (DIHS) due to human herpes virus 6 infection or to allergy-mediated viral reactivation? Report of a case and literature review. BMC Infect Dis 2010;10:49.
[Google Scholar]
|
| 11. |
Kardaun SH, Sekula P, Allanore LY, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): An original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol 2013;169:1071-80.
[Google Scholar]
|
| 12. |
Chen YC, Chiu HC, Chu CY. Drug reaction with systemic symptoms. A Retrospective study of 60 cases. Arch Dermatol 2010;146:1373-9.
[Google Scholar]
|
| 13. |
Eshki M, Allanore L, Musette P, Milpied B, Grange A, Guillaume JC, et al. Twelve-Year analysis of severe cases of drug reaction with Eosinophilia and systemic symptoms a cause of unpredictable multiorgan failure. Arch Dermatol 2009;145:67-72.
[Google Scholar]
|
| 14. |
Sasidharanpillai S, Riyaz N, Khader A, Rajan U, Binitha MP, Arunkumar G. Study on reactivation of herpes family of viruses in cutaneous adverse drug reactions. Indian J Dermatol Venereol Leprol 2013;79:725.
[Google Scholar]
|
| 15. |
Riyaz N, Sarita S, Arunkumar G, Sabeena S, Manikoth N, Sivakumar CP. Drug-induced hypersensitivity syndrome with human herpesvirus-6 reactivation. Indian J Dermatol Venereol Leprol 2012;78:175-7.
[Google Scholar]
|
Fulltext Views
5,347
PDF downloads
2,224





