Translate this page into:
Dupilumab as monotherapy for bullous pemphigoid with multiple underlying diseases: A report of two cases
Corresponding author: Miss. Shi Wu, Department of Dermatology, The First Affiliated Hospital of Jinan University, Jinan University, & Jinan University Institute of Dermatology,No. 601, West Huangpu Avenue, Guangzhou-510630, China. wushi.good@163.com
-
Received: ,
Accepted: ,
How to cite this article: Chen J, Huang H, Deng L, Wu S. Dupilumab as monotherapy for bullous pemphigoid with multiple underlying diseases: A report of two cases. Indian J Dermatol Venereol Leprol 2023;89:888-90.
Dear Editor,
Bullous pemphigoid (BP) is an autoimmune blistering disorder that occurs mostly in elderly individuals and manifests as tense blisters over normal skin or overlying red plaques or patches. The tense blisters do not rupture easily and the Nikolsky sign is negative. Traditional therapies include topical or systemic corticosteroids, immunosuppressants and anti-inflammatory antibiotics. Studies have shown that autoimmune responses dominated by Th2 cells play a key role in the pathogenesis of BP. Dupilumab is a fully human monoclonal Ig4 antibody that blocks interleukin (IL)-4 and IL-13, key drivers of type 2 helper T-cell (Th2)-mediated inflammation,1 providing a theoretical basis for its use in BP treatment.
We report two cases of BP patients with senility and multiple underlying diseases, who were treated with dupilumab alone. Detailed information about the two patients is provided in Table 1. The clinical manifestations of these patients included tense blisters on an erythematous base on the trunk and extremities, and oedema of the distal extremities [Figure 1]. They had no mucosal involvement. The Nikolsky sign and the bulla spread sign were negative. Histopathology of the lesions showed subepidermal blisters with eosinophil infiltration. In the first patient, direct immunofluorescence demonstrated linear deposition of C3 in the basement membrane zone; in the second patient, ELISA showed elevated serum levels of BP180 and BP230 antibodies [Table 2]. Both cases fulfilled the diagnostic criteria for BP, in keeping with the 2022 EADV guidelines.2
| Number | Gender | Age | Duration of blisters and erythema | Systemic medications for BP before dupilumab | Comorbid diseases | Time for blisters to start drying up | Time for erythema to begin to fade | Time for itching relief | BPDAI score | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | |||||||||
| 1 | M | 66 | 4 months | None | Diabetes, hypertension, chronic kidney failure, history of cerebral infarction | 4 days | 2 days | 3 days | 67 | 23 |
| 2 | M | 79 | 3 months | None | Diabetes, hypertension, asthma, history of cerebral infarction | 3 days | Enlargement of the area on the second day after the first injection, which faded on its own | 4 days | 60 | 34 |
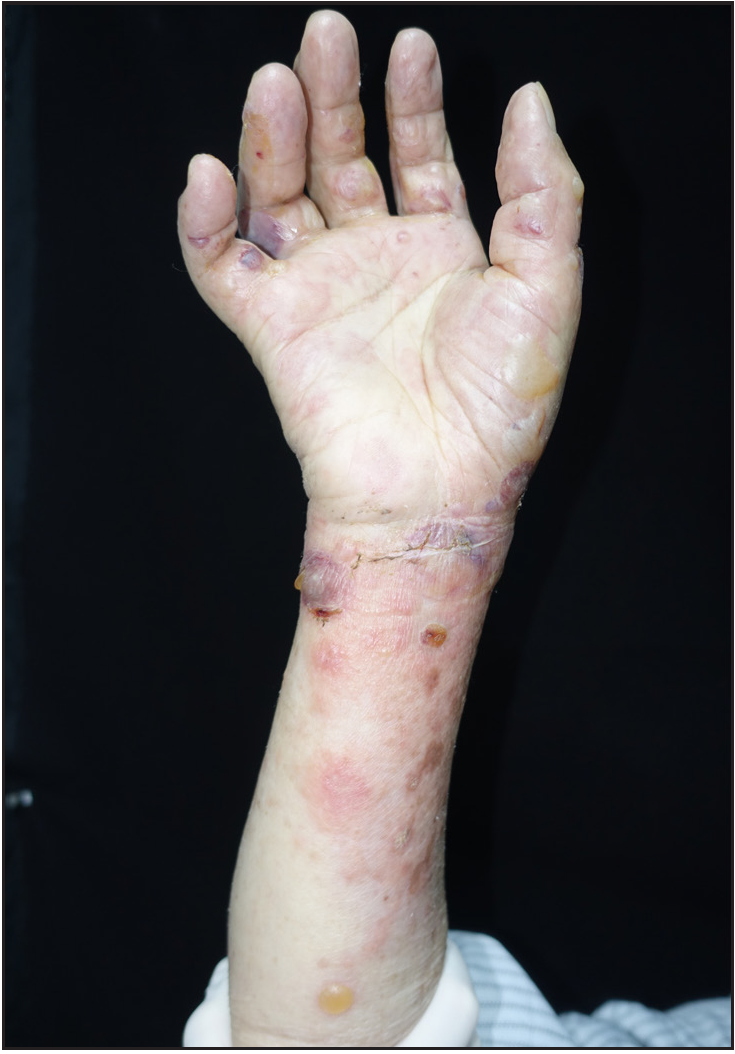
- The lesions of the second patient before dupilumab treatment
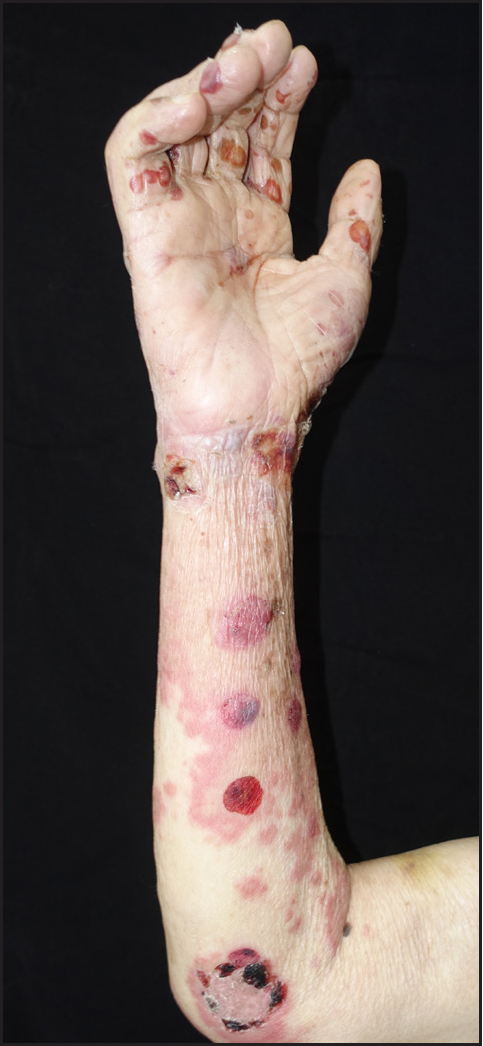
- Patient 2 developed erythema enlargement of the forearm 7 days after use of dupilumab with significant reduction of oedema and no bullae
| Number | Levels of IgE (IU/mL) | Eosinophils counts (×10 9 /L) | BP180, BP230 antibodies | ||
|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | ||
| 1 | 1994.00 | 1207.00 | 4.06 | 7.77 | BP180 antibodies (–) |
| BP230 antibodies (–) | |||||
| 2 | 5262 | 4127 | 1.93 | 2.06 | BP180 antibodies: >150 U/mL |
| BP230 antibodies: 54.67 U/mL | |||||
As the two patients were elderly and had multiple underlying diseases, it was believed that traditional systemic therapy might aggravate their underlying diseases, along with other risks. In addition, both patients had severe pruritus and significantly increased IgE levels and eosinophil counts [Table 2]. Based on recommendations of the Chinese Dermatologist consensus on the diagnosis and treatment of autoimmune subepidermal bullous diseases (2022),3 dupilumab alone was selected for treatment. After obtaining written informed consent from the patients and their families, the two patients received loading doses of dupilumab (Dupixent®, Regeneron Pharmaceuticals, Terrytown, NY; Sanofi Genzyme, Cambridge, MA) 600 mg subcutaneously; followed by subcutaneous injections of dupilumab 300 mg every other week for a total of 16 weeks.
The oedema of both patients began to decrease on the day after the first injection, with complete subsidence in 3 days [Figure 2], suggesting another potential role for dupilumab in the treatment of BP symptoms other than itching and blisters.
The second patient developed enlargement of the erythematous areas on the extremities a day after the first injection of dupilumab. Allergic contact dermatitis was ruled out because the patient had not been exposed to any possible allergenic substances. To our knowledge, such a phenomenon after dupilumab injection in BP patients has not been reported thus far. The erythema resolved on its own without intervention. This patient did not experience similar symptoms after the subsequent injections of dupilumab [Figure 3].
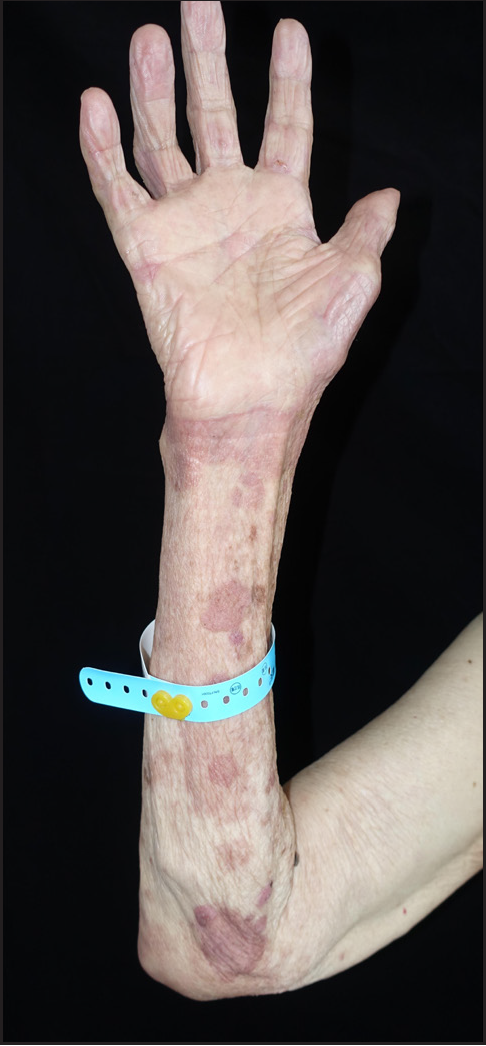
- Blisters and bullae on the forearms are dry after 4 courses of dupilumab therapy in patient 2
At follow-up visits every 2 weeks, these patients had no exacerbations of their original disease and no adverse effects. After 16 weeks of full treatment follow-up, none of the patients showed signs of relapse. The present cases suggest the high safety of dupilumab for BP. A critical step in the pathogenesis of BP is damage to the basement membrane zone mediated by tissue-bound and circulating IgG autoantibodies.4 The Th2 inflammatory response is closely linked to the production of antibodies against the hemidesmosomal complex, which primarily includes BP230 and BP180. Regarding the mechanisms of action of dupilumab, it not only inhibits the Th2 inflammatory response but also indirectly downregulates IgE secretion and eosinophil activity to reduce IL-31 secretion, which helps relieve pruritus symptoms in the patients.5 There have been several case reports on the use of dupilumab to treat BP and the largest series so far was is the multi-institutional study reported by Abdat et al.6 In the two cases reported herein, dupilumab showed significant efficacy in BP patients who had severe pruritus with elevated IgE and eosinophilia, and the drug had a significant ameliorating effect on the blisters and oedema of BP. Thus, dupilumab is expected to be an effective treatment for elderly BP patients with multiple underlying diseases, who are not able to undergo conventional treatment, especially those with the above-described symptoms; furthermore, dupilumab can be used as a single drug to treat BP. However, these findings need to be confirmed by further studies with larger sample size.
Acknowledgments
We would like to thank American Journal Experts for performing language editing of this manuscript (Reference: C72YKPC4).
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
GuangDong Basic and Applied Basic Research Foundation (No. 2020A1515110115)
Conflicts of interest
There are no conflicts of interest.
References
- Dupilumab use in dermatologic conditions beyond atopic dermatitis - a systematic review. J Dermatolog Treat. 2021;32:19-28.
- [CrossRef] [PubMed] [Google Scholar]
- Updated S2 K guidelines for the management of bullous pemphigoid initiated by the European Academy of Dermatology and Venereology (EADV) J Eur Acad Dermatol Venereol. 2022;36:1689-1704.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus on the diagnosis and treatment of autoimmune subepidermal bullous diseases. Chin J Dermatol. 2022;55:1-11.
- [Google Scholar]
- Structural proteins of the dermal-epidermal junction targeted by autoantibodies in pemphigoid diseases. Exp Dermatol. 2017;26:1154-62.
- [CrossRef] [PubMed] [Google Scholar]
- Th1, Th2 and Th3 cytokines in the pathogenesis of bullous pemphigoid. J Dermatol Sci. 2002;30:116-28.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab as a novel therapy for bullous pemphigoid: A multicenter case series. J Am Acad Dermatol. 2020;83:46-52.
- [CrossRef] [PubMed] [Google Scholar]





