Translate this page into:
Eccrine syringofibroadenomatosis of the reactive subtype occurring in chronic poorly-controlled psoriasis
Correspondence Address:
Yee Kiat Heng
National Skin Centre, 1 Mandalay Road, Singapore 308205
Singapore
| How to cite this article: Heng YK, Pan JY, Tan SH, Tey HL. Eccrine syringofibroadenomatosis of the reactive subtype occurring in chronic poorly-controlled psoriasis . Indian J Dermatol Venereol Leprol 2014;80:534-536 |
Abstract
Eccrine syringofibroadenomatosis (ESFA) is a rare adnexal tumor with acrosyringeal differentiation. Clinically, it can be mistaken for granulomatous infections or malignancies such as squamous cell carcinoma. Despite the rarity of the condition, we recently encountered two cases of the reactive subtype, which occurred in patients with poorly controlled chronic psoriasis. Both patients presented with long-standing, thick verrucous lesions on the lower legs. The diagnosis was made after histological examination and exclusion of infectious and neoplastic disorders. As this is a reactive disorder, management is focused on treating the underlying condition. Unfortunately, psoriasis was difficult to manage in both our patients and they defaulted further treatment. It is important to recognize ESFA as it can be confused with infectious or malignant disorders.INTRODUCTION
Eccrine syringofibroadenomatosis (ESFA) of Mascaro is a rare adnexal tumor which shows acrosyringeal ductal differentiation. There are five subtypes, one of which is known to be reactive to chronic inflammatory or neoplastic dermatoses. We report two patients with poorly-controlled psoriasis and reactive ESFA seen in the National Skin Centre, Singapore.
CASE REPORTS
Case 1
A 49 year-old Chinese man suffering from chronic erythrodermic psoriasis presented with asymptomatic clusters of thick, irregular, verrucous nodules on both shins [Figure - 1] which had been slowly enlarging for a year. There was no regional lymphadenopathy. His psoriasis of 20 years duration had limited response to systemic oral treatments (methotrexate, cyclosporine and acitretin) and phototherapy and he was unable to afford long-term treatment with adalimumab.
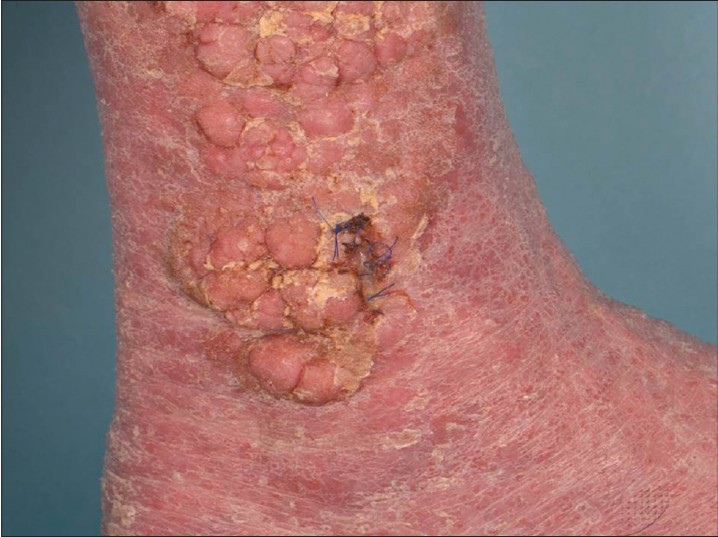 |
| Figure 1: Cluster of multiple irregular nodules on the shin |
Our differential diagnoses included squamous cell carcinoma (in view of previous phototherapy), infectious granulomatous disorders such as tuberculosis verrucosa cutis (in view of previous anti-tumor necrosis factor therapy), and extensive verruca vulgaris.
Histopathological examination of one of the nodules revealed thin anastomosing strands of epithelial cells forming a lattice on the undersurface of the epidermis [Figure - 2]a. Eccrine ducts were seen within the epithelial strands [Figure - 2]a inset. Immunohistochemistry showed positive carcinoembryonic antigen (CEA) staining of cells lining the eccrine ducts [Figure - 2]b.
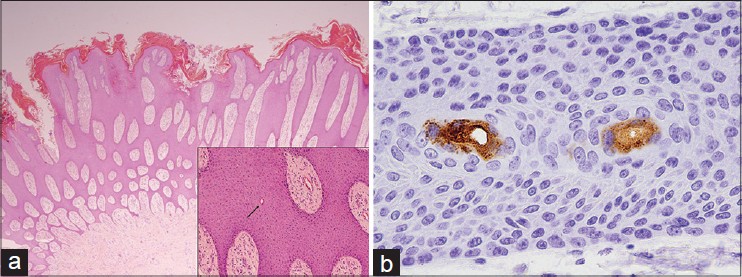 |
| Figure 2: (a) Thin anastomosing strands of epithelial cells forming a lattice on the undersurface of the epidermis. (H and E ×12.5). Inset shows eccrine ducts (arrow) within the epithelial strands. (H and E ×100). (b) Immunohistochemical staining confirmed that cells lining the ducts were of eccrine origin. (CEA ×400) |
He was diagnosed with bilateral reactive ESFA secondary to chronic erythrodermic psoriasis. Adalimumab was re-initiated with financial assistance (loading dose of 80 mg and then 40 mg every other week), but it failed to control his psoriasis after 6 months of treatment. He subsequently switched to homeopathic treatment, but no significant improvement in psoriasis or ESFA was seen.
Case 2
A 43-year-old Indian male presented with firm indurated verrucous plaques studded with numerous fleshy papulo-nodules on his lower limbs [Figure - 3] which had been slowly increasing in size for the past 2 years. These lesions were otherwise asymptomatic and not associated with regional lymphadenopthy. In addition, he had thick psoriatic plaques on the trunk and arms. He had suffered from chronic plaque psoriasis for many years for which he had been using topical medications intermittently with poor treatment compliance.
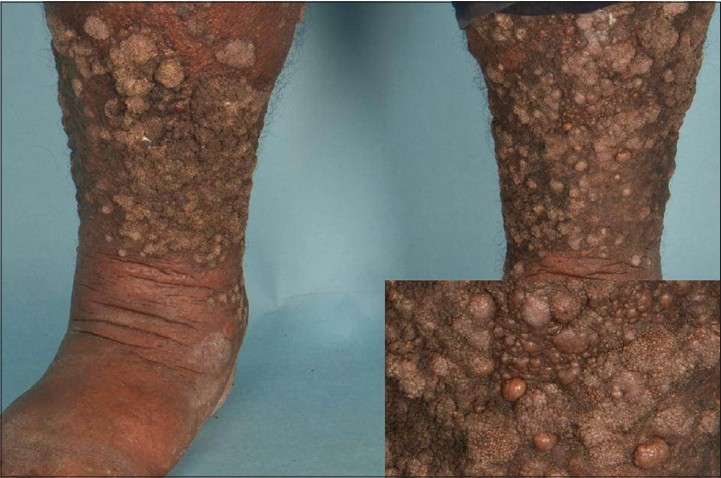 |
| Figure 3: Fleshy nodules of ESFA (inset) on a background of verrucous psoriasis and mild lymphedema |
His other medical problems included diabetes mellitus, hypertension, hyperlipidemia, renal tubular acidosis type 4 with hyperkalemia, and squamous cell carcinoma of the penis which had been excised 2 years earlier.
Histological examination of one of the papulo-nodules revealed irregular epidermal hyperplasia, with thin elongated strands of epithelial cells with ductal structures within them (similar to case 1). He was diagnosed with ESFA secondary to chronic plaque psoriasis.
The patient declined further follow-up and treatment.
DISCUSSION
ESFA, first described by Mascaro in 1963, [1] is a histological entity that may present clinically in several ways. Starink classified the disease into four types:solitary, multiple and associated with ectodermal dysplasia, multiple without associated cutaneous findings and non-familial unilateral linear ESFA. [2] In 1997, French proposed a fifth reactive subtype. [3]
Solitary ESFA presents as a non-hereditary verrucous growth in middle-aged and elderly individuals. Multiple ESFA presents with multiple erythematous rough papules on the palms and soles and may be associated with ectodermal dysplasias such as Schopf syndrome (cystic lesions of the eyelids, hypotrichosis, hypodontia and nail hypoplasia) or Clouston syndrome (palmoplantar hyperkeratosis, patchy alopecia and nail dystrophy with normal teeth and sweating). Human papilloma virus type-10 DNA has since been detected in lesions of multiple ESFA associated with Clouston syndrome. [4] Multiple ESFA can also present without associated cutaneous findings.
Our two cases belong to the reactive subtype, which represents epithelial change in response to other inflammatory or neoplastic dermatoses. Reactive ESFA has been reported in association with chronic ulceration or repeated trauma in diabetes mellitus, leprous neuropathy, burn scars, venous stasis, nail trauma, bullous pemphigoid, erosive palmoplantar lichen planus, peristomal dermopathy, sebaceous nevus, epithelioid hemangioendothelioma and squamous cell carcinoma.
We have previously reported reactive ESFA associated with psoriasis and venous stasis [5] and also occurring in a neuropathic foot associated with leprosy, which showed spontaneous partial involution over time. [6]
The exact pathogenesis of reactive ESFA is unknown, but both our cases had long-standing poorly-controlled psoriasis, which is a condition characterized by chronic inflammation and marked changes in keratinocyte growth and differentiation. The molecular mechanisms behind this alteration in epidermal growth and differentiation are incompletely understood, though cytokines, such as interleukin (IL)-1a, IL-17, IL-23, transforming growth factor (TGF)-α and vascular endothelial growth factor have been implicated. [7]
Derangements in the Wnt/B-catenin signaling pathway are reported in the pathogenesis of both psoriasis [8] and eccrine tumors. [9] This signaling pathway is important in cell differentiation and proliferation and is involved in development of malignancies, such as colorectal cancer. [10] There is possibly loss of inhibition or overexpression of signaling within this pathway in both psoriasis and eccrine tumors via a common molecule, such as TGF-α, and it is tempting to conjecture that development of ESFA may be linked to psoriasis in this way. However, this particular signaling pathway is a complex one with multiple levels of control and there is insufficient evidence to prove this hypothesis currently.
ESFA is usually clinically benign though malignant change has been reported in at least five cases of non-reactive ESFA. [11] In cases of reactive ESFA, there have been no reports of malignant transformation and partial spontaneous involution has been reported in 1 case. [6] Hence, it may be prudent to monitor these cases and treat the underlying cause, instead of aggressive surgical removal.
While reactive ESFA remains rare, it mimics malignancy and atypical mycobacterial and deep fungal infections clinically, and its occurrence warrants more aggressive treatment of the underlying condition.
| 1. |
Mascaro JM. Considerations sur les tumeurs fibro-epitheliales. Le syringofibroadenome eccrine. Ann Dermatol Syphiligr 1963;90:146-53.
[Google Scholar]
|
| 2. |
Starink TM. Eccrine syringofibroadenoma: Multiple lesions representing a new cutaneous marker of the Schopf syndrome, and solitary nonhereditary tumors. J Am Acad Dermatol 1997;36:569-76.
[Google Scholar]
|
| 3. |
French LE. Reactive eccrine syringofibroadenoma: An emerging subtype. Dermatology 1997;195:309-10.
[Google Scholar]
|
| 4. |
Carlson JA, Rohwedder A, Daulat S, Schwartz J, Schaller J. Detection of human papillomavirus type 10 DNA in eccrine syringofibroadenomatosis occurring in Clouston's syndrome. J Am Acad Dermatol 1999;40:259-62.
[Google Scholar]
|
| 5. |
Tey HL, Tan SH. Reactive eccrine syringofibroadenomatosis associated with venous stasis and plaque psoriasis. Acta Derm Venereol 2008;88:82-4.
[Google Scholar]
|
| 6. |
Tey HL. Characterizing the nature of eccrine syringofibroadenoma: Illustration with a case showing spontaneous involution. Clin Exp Dermatol 2009;34: e66-8.
[Google Scholar]
|
| 7. |
Coimbra S, Figueiredo A, Castro E, Rocha-Pereira P, Santos-Silva A. The roles of cells and cytokines in the pathogenesis of psoriasis. Int J Dermatol 2012;51:389-95.
[Google Scholar]
|
| 8. |
Gudjonsson JE, Johnston A, Stoll SW, Riblett MB, Xing X, Kochkodan JJ, et al. Evidence for altered Wnt signaling in psoriatic skin. J Invest Dermatol 2010;130:1849-59.
[Google Scholar]
|
| 9. |
Im M, Kim DH, Park JS, Chung H, Lee Y, Kim CD, et al. Alteration of the β-catenin pathway in spiradenoma. J Cutan Pathol 2011;38:657-62.
[Google Scholar]
|
| 10. |
Luo J, Chen J, Deng ZL, Luo X, Song WX, Sharff KA, et al. Wnt signaling and human diseases: What are the therapeutic implications? Lab Invest 2007;87:97-103.
[Google Scholar]
|
| 11. |
Bjarke T, Ternesten-Bratel A, Hedblad M, Rausing A. Carcinoma and eccrine syringofibroadenoma: A report of five cases. J Cutan Pathol 2003;30:382-92.
[Google Scholar]
|
Fulltext Views
3,639
PDF downloads
3,095





