Translate this page into:
Effect of chronicity and treatment on in vivo morphology and density of dermatophytes
2 Department of Dermatology, Government Medical College, Kozhikode, Kerala, India
Correspondence Address:
K Devi
Department of Dermatology, Government Medical College, Kozhikode, Kerala
India
| How to cite this article: Sunil G, Devi K, Ambooken B, Asokan N. Effect of chronicity and treatment on in vivo morphology and density of dermatophytes. Indian J Dermatol Venereol Leprol 2020;86:736-738 |
Sir,
There has been an alarming increase in the prevalence and change in clinical pattern of dermatophytoses over the past 4-5 years across India.[1] It is not clear if different clinical patterns of dermatophytosis are associated with any changes in fungal morphology.
We did a comparative cross sectional study in the Department of Dermatology, Government Medical College, Thrissur, Kerala to determine if there is any difference in the morphology of fungal hyphae and density of dermatophytes as seen on 10% potassium hydroxide (KOH) smear in different clinical types of tinea corporis and tinea cruris. Of the 143 consecutively attending patients, we selected 107 (74.8%) patients in whom dermatophytes could be demonstrated on KOH smear. They were divided into three groups: Group A (n = 32) consisting of patients who had received no prior antifungal therapy (naive cases), Group B (n = 51) consisting of patients who had used topical agents containing corticosteroids (n = 19); who had stopped antifungal therapy before the recommended duration (n = 12); or those with diabetes mellitus (n = 17); HIV (n = 1) or those on systemic immunosuppressants (n = 2) and Group C (n = 24) consisting of patients who had persistent infection despite taking antifungal therapy (systemic/topical) at the recommended dose and duration [defined as: tab fluconazole 150mg once or twice weekly for a minimum of 4 weeks, tab terbinafine 250mg per day for 2-4 weeks, tab itraconazole 100mg twice daily for 2- 4 weeks and tab griseofulvin 250-500 mg twice daily for 2-4 weeks].
The KOH positive smears were further examined using a binocular microscope with an ocular micrometre to assess the width of the hyphae. Each small division of the micrometer corresponded to 2.5 micrometre.
Density of hyphae and spores were also assessed and graded as follows:
Scanty = 1-10 hyphae or spores per 10 fields;
Mild = 1-10 hyphae or spores in every field;
Moderate = >10, but countable number of hyphae or spores/field;
Abundant = uncountable hyphae or spores in a field
If only spores were present, their density was graded as follows:
Few = 1- 10;
Moderate = >10, but countable;
Abundant – uncountable
Age of the patients ranged from 8-70 years (mean = 36.13 ± 16.9). Females outnumbered males (67:40). Seventy three [68.2%] patients had lesions involving more than 10% of body surface area. Papulosquamous was the most common [n = 73; 62.8%] morphological type.
Forty eight (n = 48; 44.9%) patients had scanty and 48 (44.9%) had moderate density of hyphae on KOH smear. Though the density of hyphae was slightly more in Groups B and C, the difference was not statistically significant [P = 0.71] [Table - 1]. The density of spores was low in the majority (n = 70; 65.4%) of patients and was not significantly different among three groups (P = 0.624). Hyphal thickness measured under ocular microscope was less than 2.5 micrometre in the majority of patients (n = 56; 52.3%). Broader hyphae (width more than 2.5 micrometre) was more frequent in Group B, compared to the other two groups (P = 0.001). Among the 15 patients in whom we identified an increased hyphal width, 14 (93.3%) belonged to group B.
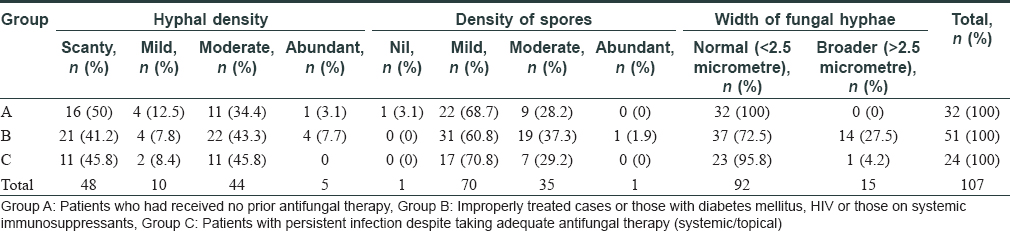
Increased density of fungal hyphae has been described in dermatophytosis in the presence of immunosuppression,[2] though our study did not support that. The notable finding in our study was the increased fungal width and not increased density of fungal hyphae in cases which were improperly treated particularly with corticosteroids, or those with underlying diabetes mellitus or immunosuppression. Environmental, host and fungal factors can influence growth of dermatophytes.[3],[4] External agents can affect the rate of growth of dermatophytes and result in changes in the morphology of hyphae.[5] Application of topical corticosteroids have been shown to decrease the level of free fatty acids and cholesterol and ceramides, impair the barrier function of skin and result in epidermal atrophy and xerosis.[6]
The limitations of our study include the cross sectional design, selection bias due to hospital based recruitment of cases, lack of a prior sample size calculationand limited precision of the instrument. Studies with case control design may be undertaken with sample size calculated based on our findings in future. The effect of corticosteroids on fungi grown in culture may be studied. Studies exploring the mechanism by which topical or systemic immunosuppression cause increased hyphal width also may be undertaken.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Acknowledgment
We thank Mrs Subi T, lab technician for her assistance in evaluating the fungal width and density of spores and hyphae.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Panda S, Verma S. The menace of dermatophytosis in India: The evidence that we need. Indian J Dermatol Venereol Leprol 2017;83:281-4.
[Google Scholar]
|
| 2. |
Piérard GE, Piérard-Franchimont C, Hermanns-Lê T, Hermanns JF, Delvenne P. Dermatophyte growth in glabrous skin dermatophytosis of immunocompromised hosts. J Med Diagn Methods 2015;4:186.
[Google Scholar]
|
| 3. |
Esquenazi D, Alviano CS, de Souza W, Rozental S. The influence of surface carbohydrates duringin vitro infection of mammalian cells by the dermatophyte Trichophyton rubrum. Res Microbiol 2004;155:144-53.
[Google Scholar]
|
| 4. |
Ninomiya J, Ide M, Ito Y, Takiuchi I. Experimental penetration of Trichophyton mentagrophytes into human stratum corneum. Mycopathologia 1998;141:153-7.
[Google Scholar]
|
| 5. |
Ghahfarokhi MS, Goodarzi M, Abyaneh MR, Al-Tiraihi T, Seyedipour G. Morphological evidences for onion-induced growth inhibition of Trichophyton rubrum and Trichophyton mentagrophytes. Fitoterapia 2004;75:645-55.
[Google Scholar]
|
| 6. |
Del Rosso JQ, Cash K. Topical corticosteroid application and the structural and functional integrity of the epidermal barrier. J Clin Aesthet Dermatol 2013;6:20-7.
[Google Scholar]
|
Fulltext Views
2,351
PDF downloads
994





