Translate this page into:
Effectiveness and safety of 0.5% timolol solution in the treatment of pyogenic granuloma: A randomized, double-blind and placebo-controlled study
Corresponding author: Prof. Nilay Kanti Das, Professor, Dept. of Dermatology, Bankura Sammilani Medical College, Kenduadihi, Bankura – 722102, West Bengal. Email: drdasnilay@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Patra AC, Sil A, Ahmed SKS, Rahaman S, Mondal N, Roy S, et al. Effectiveness and safety of 0.5% timolol solution in the treatment of pyogenic granuloma: A randomized, double-blind and placebo-controlled study. Indian J Dermatol Venereol Leprol 2022;88:500-8.
Abstract
Introduction
Pyogenic granulomas are benign vascular lesions of the skin and mucosa which are often a source of concern because of their recurrent bleeding even with minimal trauma. Current treatment for pyogenic granuloma is ablative; no medical therapy is standardized to date. Timolol, due to its vasoconstrictive effect, vascular growth factor inhibition and apoptosis promotion properties, is a potential therapeutic option. Objectives: To assess the effectiveness and safety of topical timolol in the treatment of pyogenic granulomas.
Methods
A two-centre, double-blind and placebo-controlled trial (Registration CTRI/2019/04/018581) was conducted. Patients of either sex were recruited with pyogenic granuloma lesions of less than eight weeks duration. Topical treatment with 0.5% timolol or matching glycerin placebo was continued for six weeks. Changes in color, size, bleeding tendency, physicians’ and patients’ global assessments and adverse events were assessed.
Results
Forty subjects were randomized between the two groups which were comparable in age, sex, duration of illness and baseline lesion size.Significant improvement was noted with timolol, with color change from first follow-up onwards and lesion size reduction from second follow-up onward. Patients’ assessment of bleeding tendency also showed imrovement from the second visit onward. Between-group comparison showed significant difference with respect to percentage reduction in size (timolol 40.9%, placebo 3.4%; P = 0.002). Rescue treatment (electrosurgery) was required in five patients on placebo and in one in the timolol group (P = 0.182). Complete resolution occurred in 2 (10%) patients with timolol and in no patients on placebo (P = 0.231). Limitations: We observed effects of treatment for only six weeks.
Conclusion
Topical timolol may be a treatment option for early pyogenic granulomas but complete resolution is unlikely in six weeks. Studies of longer duration are required to assess resolution and recurrence rates.
Keywords
Pyogenic granuloma
randomized controlled trial
timolol
Introduction
Pyogenic granuloma or lobular capillary hemangioma is a benign acquired vascular lesion of the skin and mucosa. The exact etiology of pyogenic granuloma is not known. Various factors such as trauma, hormonal effects, production of angiogenic growth factors, drugs and viral causes have been suggested as possible contributors to the etiopathogenesis.1-3 Pyogenic granulomas tend to occur more commonly in children and young adults. Though benign, pyogenic granulomas are often a source of concern for the patient or caregivers because of their recurrent bleeding associated with trauma. Further, pyogenic granuloma lesions on the face can be of cosmetic concern.4,5
Various treatment options have been tried for pyogenic granulomas. The physician has to consider different aspects such as efficacy of the treatment method, number of sittings required, and risks of scarring and recurrence in selecting an option.4 Choices include surgical excision,3,6 curettage, shave excision with cautery, cryotherapy,7,8 CO2 laser,9 pulsed dye laser10 and sclerotherapy.11 Surgical excision, curettage and cautery are associated with lower chances of recurrence but can be associated with scarring to some degree.3,6-8 Lasers and sclerotherapy may require multiple sittings. Sclerotherapy has been shown to be associated with minimal scarring.9,11 Finally, simple follow-up of a pyogenic granuloma (expectant management) may yield spontaneous regression between 6 and 18 months, though with mild scarring.4
A recent case series described seven children with pyogenic granulomas successfully treated with beta-blockers, of whom six were given topical timolol.5 The exact mechanism of action of beta-blockers on vascular lesions is not known. In the treatment of infantile hemangiomas, it has been proposed that the blocking of vasoconstriction and beta-adrenergic receptor expression by timolol causes the observed softening of these vascular tumours.12 It is hypothesized that since similar underlying factors may be involved in infantile hemangiomas and pyogenic granulomas, beta-blockers may be equally effective in both cases.12
The endothelial cells in pyogenic granulomas express CD34, ICAM-1 and VCAM-1, associated with increased microvascular density.13 The Ras pathway has been previously implicated in angiogenesis and vascular proliferation through hypoxia-inducible factor 1-alpha (HIF1a) and vascular endothelial growth factor (VEGF).14 It is postulated that beta-blockers work by inhibiting hypoxia-inducible factor 1-alpha and vascular endothelial growth factor, leading to vasoconstriction, a decrease in angiogenesis and induction of apoptosis of the endothelial cells involved.15 Timolol, a nonselective beta-blocker with potent peripheral action and five times more potent than propranolol, may therefore work in pyogenic granulomas by these mechanisms16 If effective, topical beta-blockers could prevent unnecessary surgical intervention and scarring in pyogenic granulomas, especially in pediatric cases. We undertook the present study with the objectives of assessing the effectiveness and safety of topical timolol in pyogenic granulomas of recent onset (less than eight weeks).
Materials and Methods
The study was designed as a double-blind, randomized (1:1) and placebo-controlled trial conducted at two centres (Dermatology Outpatient Departments of Bankura Sammilani Medical College, Bankura and Medical College, Kolkata in West Bengal, India). It was initiated after obtaining the approval of the Institutional Ethics Committees of the abovementioned institutions and registering in the Clinical Trial Registry of India (registration number CTRI/2019/04/018581). Patients were recruited over four months and followed up for six weeks each.
Sample size and randomization
The calculated sample size was 40 participants, calculated considering 5% Type 1 error probability, 80% power and effect size of 51% difference in proportion showing complete resolution of pyogenic granulomas (from an unpublished pilot study on four patients). Simple, unstratified, unbalanced randomization was done using WINPEPI software (ETCETERA version 2.32.) with a 1:1 allocation ratio.
Inclusion and exclusion criteria
Pyogenic granulomas were diagnosed clinically. Patients of either sex in the age range of 2–65 years having pyogenic granulomas for around two months or less’ were included with their written informed consent. Pregnant or lactating women were excluded as were those who had used any other treatment modality previously. Other exclusion criteria were co-morbidities such as sinus bradycardia, heart block, bronchial asthma, chronic obstructive pulmonary disease, persistent hypoglycemia, or use of systemic medication (e.g., systemic beta-blockers, calcium channel blockers and quinidine) that precluded the use of timolol.
Study medications
Timolol maleate 0.5% (TIMANOL 0.5% sterile eye drops, Sunways (India) Pvt. Ltd; Batch No. 1801; Expiry date: 05/2020) was applied locally twice daily by the patient or their parent for six weeks. One to two drops were advised at each application according to the size of the lesion. Glycerin (B.D. Pharmaceutical Works Pvt. Ltd; Batch No. G5043; Expiry date: 10/19) was used as a placebo due to its similar appearance and consistency. Glycerin was also applied locally twice daily for six weeks to maintain blinding.
Blinding and allocation concealment
Labels on the vials of timolol were removed, vials wrapped with white adhesive tape and labeled as study medication. Glycerin was poured into similar looking vials (used and expired timolol vials thoroughly washed beforehand) and also covered with white adhesive tape by a person unrelated to the study. Thus, patient blinding was achieved. Vials were placed in sequentially numbered sealed opaque envelopes and dispensed by a physician as per the randomization list. Thus, allocation concealment was done. The dispensing and assessing physicians were different and the assessing physicians were unaware of the medication received. Thus, investigator blinding was achieved.
Study parameters
The effectiveness parameters were reduction in size, change in color and complete resolution of the pyogenic granuloma. Change in the maximum diameter of the lesion was recorded in millimeters at each visit. Improvement in color from red to dark to normal skin color was recorded subjectively in terms of four grades: Grade 1 was complete improvement and return to skin color and Grade 4, no change with the lesion remaining red. Patients’ Global Assessment according to bleeding tendency (five-point Likert scale; Grade 1 – Excellent, Grade 2 – Very good, Grade 3 – Good, Grade 4 – Poor and Grade 5 – No change) and Physicians’ Global Assessment (five-point Likert scale; Grade 1 – Excellent, Grade 2 – Very good, Grade 3 – Good, Grade 4 – Poor and Grade 5 – No change) were also outcome measures. Safety parameters assessed included changes in laboratory parameters at the end of the treatment, patient-reported adverse events and those elicited by the clinicians.
Visits and follow-ups
In the initial visit, patients were recruited after screening for inclusion and exclusion criteria, and randomized to the two treatment arms. Three follow-up visits were done, at intervals of two weeks. Effectiveness and safety parameters were assessed, and study medication was dispensed according to the randomization list at the first and second follow-up visits. In the last follow-up at six weeks final outcomes were noted.
Statistical analysis
Normality testing was done by D’Agostino-rson test. Normally distributed continuous variables were compared between groups by independent samples t-test and within groups by paired t-test. Mann–Whitney U-test and Wilcoxon’s matched pairs signed rank test were employed for comparison of unpaired and paired nonparametric data. Friedman’s analysis of variance was carried out with non-parametric data for within-group repeated measures comparisons, followed by post hoc Dunn’s test. Categorical data were compared between groups by Chi-squared test or Fisher’s exact test, as appropriate. Associations between percentage reduction of size of the lesions and baseline clinico-demographic variables were quantified by Spearman’s rank correlation coefficient. MedCalc version 11.6 (Mariakerke, Belgium: MedCalc Software, 2011) software was used for statistical analysis.
Effectiveness analysis was done on a modified intention-to-treat basis with subjects reporting for at least one follow-up visit. Missing values were dealt with by the last observation carried forward strategy. For the safety analysis, all subjects who had received at least one dose of a study drug (essentially all 40 subjects) were considered.
Results
Of 47 patients screened, seven were excluded as per exclusion criteria and the remaining 40 were randomized, 22 to the timolol group and 18 to the glycerin group. [Figure 1] All randomized participants completed at least their first follow-up visit and were thus eligible for intention-to-treat analysis.
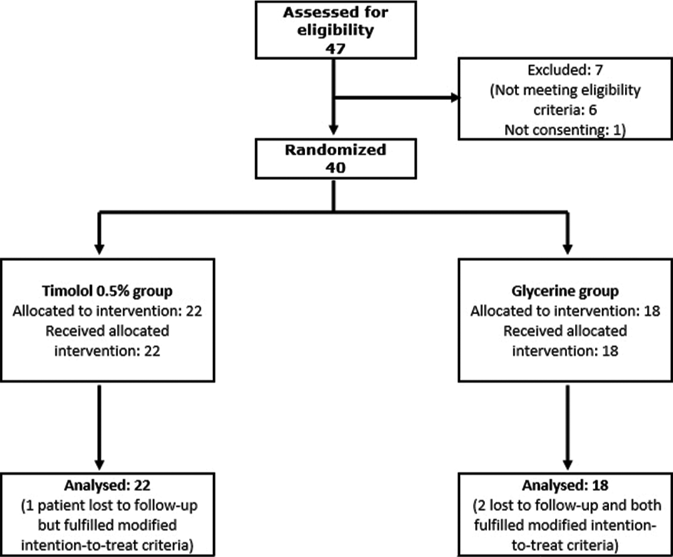
- Chart depicting the flow of participants through the study
Overall mean (± standard deviation) age of our study population was 30.2 ± 13.51 years, and the mean age was comparable between the two treatment arms (P = 0.426). Most patients were male, lacking in literacy and residing in rural areas. Mean duration of illness was 33.0 ± 12.34 days and this was also comparable between the groups (P = 0.103). Common sites of involvement were the face (27.5%), lateral nailfold (22.5%), palm or sole (22.5%) and scalp (20%). Baseline characteristics are summarized in Table 1.
| Variable | Timolol (n=22) | Glycerin placebo (n=18) | P-value (between groups) |
|---|---|---|---|
| Age (years) | |||
| Mean±SD | 28.6±11.58 | 32.1±15.68 | 0.426 |
| Median (IQR) | 25.0 (22.0–35.0) | 27.5 (20.0–45.0) | |
| Gender | |||
| Male:female | 9:13 | 9:9 | 0.798 |
| Education | |||
| Literate:illiterate | 9:13 | 4:14 | 0.360 |
| Residence | |||
| Urban:rural | 16:6 | 15:3 | 0.676 |
| Duration of illness (days) | |||
| Mean±SD | 35.9±12.55 | 29.5±11.43 | 0.103 |
| Median (IQR) | 36 (28–42) | 30 (21–42) | |
| Site of lesions | |||
| Scalp | 5 (22.7%) | 3 (16.7%) | 0.752 |
| Face including lips | 6 (27.3%) | 5 (27.7%) | |
| Lateral Nailfold | 6 (27.3%) | 3 (16.7%) | |
| Trunk | 2 (9.1%) | 1 (5.6%) | |
| Palm or Sole | 3 (13.6%) | 6 (33.3%) | |
P-value is from Student’s unpaired t-test for age and duration of illness, Fisher’s exact test for gender distribution, education and residence. Chi-square test for site of lesions. SD: Standard deviation, IQR: Inter-quartile range
As shown in Table 2, there was no statistically significant difference in lesion size at baseline (P = 0.859) between the study groups. Significant size reduction was obtained with timolol in comparison to placebo from second follow-up (week 4) onward. Friedman’s analysis of variance showed that the reduction in size with timolol was significant (P = 0.001) from first follow-up onward and continued at all follow-up visits. Interestingly, glycerin recipients also showed size reductions at first follow-up (P = 0.003) which then plateaued [Table 2]. Three patients experienced increases in lesional size, of whom 2 were on glycerin (lesions on lips and scalp) and 1 on timolol (lesion on lip) [Figures 2a and 2b].
| Visit | Timolol (n=22) | Glycerin placebo (n=18) | P-value (between groups) |
|---|---|---|---|
| Baseline | |||
| Mean±SD | 8.0±3.05 | 8.1±3.63 | 0.859 |
| Median (IQR) | 8.0 (6.0–10.0) | 6.5 (5.0–10.0) | |
| 1st follow-up | |||
| Mean±SD | 6.4±3.12 | 7.6±3.31 | 0.204 |
| Median (IQR) | 6.0 (4.0–8.0) | 6.5 (5.0–10.0) | |
| 2nd follow-up | |||
| Mean±SD | 5.3±3.48 | 7.8±3.91 | 0.045 |
| Median (IQR) | 5.0 (3.0–6.0)* | 6.5 (4.0–12.0) | |
| 3rd follow-up | |||
| Mean±SD | 4.7±3.53 | 7.6±3.95 | 0.012 |
| Median (IQR) | 3.5 (3.0–6.0)* | 6.5 (4.0–12.0) | |
| P-value (Within groups) | 0.001 | 0.003 | |
Between-groups P-values are from Mann–Whitney U-test. Within-group P-value is from Friedman’s analysis of variance; *Denotes significant reduction from baseline by post hocDunn’s test. SD: Standard deviation, IQR: Inter-quartile range

- Study participant on placebo experiencing increase in size of lesion: baseline

- Study participant on placebo experiencing increase in size of lesion: after six weeks
Table 3 summarizes the percentage size reduction in comparison to baseline. Overall reduction in size at the end of treatment at six weeks was 40.9 ± 38.1% with timolol and only 3.3 ± 40.9% with glycerin (P = 0.002). Size reduction was higher with timolol at all follow-up visits. The percentage reduction in size showed no correlation with baseline size of the lesion (Spearman’s rho +0.003, 95% CI –0.419–+0.424), duration of the lesion (rho –0.264, 95% CI –0.617–+0.174) or age of the patient (rho –0.348, 95% CI –0.671–+0.068). Site of the lesion also did not predict the percentage reduction in size and comparison of percentage reduction of lesions at the final follow-up showed no significant difference between lesions on lips and those located elsewhere (P = 0.433, Mann–Whitney test).
| Visit | Timolol (n=22) |
Glycerin placebo (n=18) |
P-value (between groups) | |
|---|---|---|---|---|
| 1st follow-up | ||||
| Mean±SD | 18.1±26.39 | 2.7±27.75 | 0.097 | |
| Median (IQR) | 17.1 (0.0–33.3) | 0.0 (0.0–20.0) | ||
| 2nd follow-up | ||||
| Mean±SD | 33.8±36.06 | 1.0±40.28 | 0.003 | |
| Median (IQR) | 38.8 (10.0–50.0)* | 5.6 (0.0–20.0) | ||
| 3rd follow-up | ||||
| Mean±SD | 40.9±38.12 | 3.3±40.85 | 0.002 | |
| Median (IQR) | 45.0 (20.0–66.7)* | 16.7 (0.0–20.0) | ||
| P-value (Within groups) | <0.001 | 0.120 | ||
Between-groups P-values are from Mann–Whitney U-test. Within group P-value is from Friedman’s analysis of variance; * denotes significant reduction from baseline by post hocDunn’s test. SD: Standard deviation, IQR: Inter-quartile range
There was a significant and sustained change in color from red to dark in the timolol group (P = 0.001) and the change was significant compared to baseline from the first follow-up onwards [Table 4]. The color change score was not significant in the glycerin placebo group (P = 0.378). The color of the lesion was also significantly different between timolol and placebo groups at all follow-up visits. [Figure 3a-c].
| Visit | Timolol (n=22) |
Glycerin placebo (n=18) |
P-value (between groups) |
|---|---|---|---|
| 1st follow-up | |||
| Mean±SD | 3.1±0.88 | 3.8±0.54 | 0.009 |
| Median (IQR) | 3.0 (3.0–4.0) | 4.0 (4.0–4.0) | |
| 2nd follow-up | |||
| Mean±SD | 2.7±1.04 | 3.8±0.54 | <0.001 |
| Median (IQR) | 3.0 (2.0–3.0)* | 4.0 (4.0–4.0) | |
| 3rd follow-up | |||
| Mean±SD | 2.4±1.09 | 3.7±0.57 | <0.001 |
| Median (IQR) | 2.0 (2.0–3.0)* | 4.0 (4.0–4.0) | |
| P-value (Within groups) | 0.001 | 0.378 | |
Between-groups P-values are from Mann–Whitney U-test. Within-group P-value is from Friedman’s analysis of variance; *Denotes significant reduction from baseline by post hocDunn’s test. SD: Standard deviation, IQR: Inter-quartile range

- Change in color of pyogenic granuloma from red to dark with reduction in size in a patient on topical timolol: baseline

- Change in color of pyogenic granuloma from red to dark along with reduction in size in a patient on topical timolol: after two weeks

- Change in color of pyogenic granuloma from red to dark along with reduction in size in a patient on topical timolol: after six weeks
By patients’ assessment, the bleeding tendency of lesions was significantly reduced with timolol (P = 0.010), an effect reported from the first follow-up visit itself [Table 5]. In contrast, there was no significant reduction of bleeding (P = 0.827) in the glycerin group. Intergroup comparisons at all follow-up visits showed statistically significant differences in favor of timolol. A similar pattern was noted with the physician’s assessment. Physicians’global assessment showed significant improvement with timolol group (p=0.012) but not with the placebo group (p=0.827). Between group comparison of the Physician’s assessment showed that the timolol group fared between than the placebo group at all follow-ups (1st follow up p=0.024, 2nd follow up p<0.001, 3rd follow-up p<0.001).
| Visit | Timolol (n=22) | Glycerin placebo (n=18) |
P-value (between groups) |
|---|---|---|---|
| 1st follow-up | |||
| Mean±SD | 3.5±1.14 | 4.4±0.85 | 0.007 |
| Median (IQR) | 4.0 (3.0–4.0) | 5.0 (4.0–5.0) | |
| 2nd follow-up | |||
| Mean±SD | 3.0±1.21* | 4.4±0.78 | <0.001 |
| Median (IQR) | 3.0 (3.0–3.0) | 5.0 (4.0–5.0) | |
| 3rd follow-up | |||
| Mean±SD | 2.8±1.36* | 4.4±0.78 | <0.001 |
| Median (IQR) | 3.0 (2.0–4.0) | 5.0 (4.0–5.0) | |
| P-value (Within groups) | 0.010 | 0.827 | |
Between-groups P-values are from Mann–Whitney U-test. Within-group P-value is from Friedman’s analysis of variance; *Denotes significant reduction from baseline by post hoc Dunn’s test. SD: Standard deviation, IQR: Inter-quartile range
Two patients (9.1%) on timolol and none on placebo showed complete resolution of their pyogenic granulomas at six weeks, though this difference was not statistically significant (P = 0.231) [Figure 4a-c, 5a-c, 6a, b]. Five (27.8%) patients on glycerin required rescue treatment in the form of electrocautery compared to only 1 (4.5%) patient on timolol (P = 0.189). There was no scarring and no adverse event was reported or noted in either group.

- Complete resolution of pyogenic granuloma on lip with topical timolol: baseline

- Complete resolution of pyogenic granuloma on lip with topical timolol: after four weeks

- Complete resolution of pyogenic granuloma on lip with topical timolol: after six weeks

- Complete resolution of pyogenic granuloma on a lateral nailfold with topical timolol: baseline

- Complete resolution of pyogenic granuloma on a lateral nailfold with topical timolol: after two weeks

- Complete resolution of pyogenic granuloma on a lateral nailfold with topical timolol: after six weeks

- Near-complete resolution of a pyogenic granuloma on the lip with topical timolol: baseline

- Near-complete resolution of a pyogenic granuloma on the lip with topical timolol: after six weeks
Discussion
Pyogenic granulomas grow rapidly and demand urgent intervention when present on areas of trauma and friction (including lips, fingers and toes). Numerous destructive treatment options are available such as cryotherapy, excision with primary closure, curettage, shave removal, electrocautery, injection of sclerosing agents and a variety of laser modalities.5,6,17 These interventions are painful and have to be performed under local anesthesia. In apprehensive children, even general anesthesia may be required. They may cause scarring and a recurrence rate as high as 15% has been reported.6 The location of the lesion can also sometimes present a challenge for surgical treatment.
Our study recorded that topical timolol led to significant reductions in size, color and bleeding tendency of pyogenic granulomas as compared to glycerin placebo. We also noted an initial size reduction in the placebo group, though this may have been due to the osmotic effect of glycerin. A percentage reduction of 40.9% with topical timolol 0.5% as opposed to only 3.4% with the placebo is an effect size strong enough to support the use of timolol in pyogenic granulomas.
A number of mechanisms have been speculated for the action of beta-blockers in pyogenic granulomas. Beta-adrenergic receptors present on vascular endothelial cells mediate peripheral vasodilation, activation of proangiogenic factors such as vascular endothelial growth factor and basic fibroblast growth factor, and inhibition of endothelial cell apoptosis.18 It is hypothesized that vasoconstriction of blood vessels within the lesion leads to size reduction as noted in infantile hemangiomas. Longer treatment may lead to regression, presumably because of inhibitory effects on vascular growth factors and promotion of apoptosis.5 However, it must be noted that beta-adrenergic receptors are only weakly expressed in pyogenic granulomas and in only 50% of them, as opposed to their strong expression in infantile hemangiomas.12
Previous studies of timolol in pyogenic granulomas were descriptive studies with no comparison groups. These are summarized in Table 6. A study by Gupta et al. included ten participants and used timolol in a dose of two drops four times daily in a case series.19 Another study by DeMaria et al. included 17 participants, but no control group.20 Ours was a randomized controlled trial with glycerin as placebo, providing stronger evidence to support the use of timolol in pyogenic granulomas. Double blinding in our study ensured that subjective biases were minimized.
| Parameters | Gupta et al. | DeMaria et al. | Lee et al. | Present study |
|---|---|---|---|---|
| Publication year | 2016 | 2018 | 2014 | 2020 |
| Number of participants | 10 | 17 | 7 | 40 |
| Type of Study | Case series | Retrospective study | Case series | Randomized controlled trial |
| Type of lesion | Pyogenic granuloma | Ocular pyogenic granuloma | Cutaneous and mucosal pyogenic granuloma in children | Pyogenic granuloma |
| Comparator arm | Nil | Nil | Nil | Topical glycerin as placebo |
| Randomization | Nil | Nil | Nil | 1:1 |
| Blinding | Nil | Nil | Nil | Double blinding |
| Duration of treatment | Three days to 2.5 months (depending on time needed to heal) | Six weeks | Six weeks to six months | Six weeks |
| Duration of non-treatment follow-up | 3rd month after beginning of therapy; 6th month in one patient | 6–27 months | Nil | Nil |
| Dose of active medication | Topical timolol 0.5% 2 drops 4 times daily | Topical timolol 0.5% twice daily | Topical timolol 0.5% or 2% topical gel forming solution two or three times a day. or Oral propranolol 2 mg/kg/day (in one patient) twice daily |
Topical timolol 0.5% twice daily |
| Outcome | • Complete response: 4 patients • Partial response: 3 patients • No response: 4 patients |
• Complete response: 15 patients • No response: 2 patients |
• Complete response: 1 patient on topical timolol, 1 patient on oral timolol • Partial response: 5 patients |
• Complete response: 2 patients • Partial response:20 patients • Percentage reduction of size: 40.9% with topical timolol versus 3.39% with placebo (glycerin) |
| Adverse events | Nil | Nil | Nil | Nil |
Our study showed complete clearance of pyogenic granulomas by six weeks in two patients and a partial response in 20 patients in six weeks. Gupta et al. found complete resolution in four patients, partial response in three patients and no response in four patients with 16 weeks of treatment.19 DeMaria et al. found complete response in 15 patients of ophthalmic pyogenic granuloma and no response in two patients after treatment for 11 months.20
In a case series by Lee et al., seven children with infantile pyogenic granulomas were treated with topical timolol 0.5%, 2%, or oral timolol (in one patient) two or three times a day for six weeks to six months.5 One patient on topical therapy had complete resolution at two months while the others on topical therapy showed a partial response. Bleeding from lesions resolved in all cases providing great satisfaction to the families of the patients. A striking observation in this study was that in some patients, pyogenic granulomas continued to regress even after cessation of the treatment. Given that pyogenic granulomas appear to lack a prolonged proliferative phase, even the short treatment may have initiated apoptosis through beta-adrenergic receptor blockade, the effect of which may have persisted for some time.5 We did not look for such an effect post-treatment in our study.
Over time the fibrous component of pyogenic granulomas increases and they then tend to persist.21,22 Timolol may therefore be more effective, in early pyogenic granulomas with a greater vascular component. We chose only recent onset pyogenic granulomas for our study with this rationale, and the results were favorable. We looked at whether lesions of shorter durations had greater size reductions with timolol, but the negative correlation between percentage size reduction and the duration of lesions was not strong. A similar weak negative correlation was noted with percentage size reduction and age of the patient.
No systemic adverse events were noted with local timolol use. This is in conformity with the earlier studies [Table 6].
Our study has its limitations. The fact that improvement had not plateaued by six weeks calls for longer studies. We only included early-onset pyogenic granulomas and the effect of topical timolol on long-standing pyogenic granulomas needs to be evaluated. Further, we had piloted the effect of timolol on four patients initially and used the results as a reference to the sample size of this study.
Despite these limitations, we can say that our study establishes the effectiveness and safety of topical timolol treatment for early (less than eight weeks) pyogenic granuloma lesions. The drug has a definite role in reducing size and bleeding tendency although complete resolution is less likely with six weeks of treatment. Timolol therefore seems a potential alternative to surgical treatment for pyogenic granulomas timolol’s role as an alternative to surgery needs to be further explored in forthcoming studies. Perhaps it could be tried as an adjunct, used before surgery to shrink lesions and reduce bleeding during the same.
Acknowledgment
The authors would like to extend thanks to Dr Saumya Chatterjee, Regional Institute of Ophthalmology, Kolkata for supplying the empty/expired vials of timolol to be used for filling the placebo.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
The study was supported by IADVL research grant.
Conflicts of interest
There are no conflicts of interest.
References
- The role of human papillomavirus in the development of pyogenic granulomas. Int J Dermatol. 1997;36:673-6.
- [CrossRef] [PubMed] [Google Scholar]
- Eruptive disseminated lobular capillary hemangioma (pyogenic granuloma) J Am Acad Dermatol. 1989;21:391-4.
- [CrossRef] [Google Scholar]
- Pyogenic granuloma in a patient on gefitinib. Acta Med Port. 2016;29:416.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical inquiries, What's the best treatment for pyogenic granuloma. J Fam Pract. 2010;59:40-2.
- [Google Scholar]
- Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonists. Pediatr Dermatol. 2014;31:203-7.
- [CrossRef] [PubMed] [Google Scholar]
- Pyogenic granuloma-the quest for optimum treatment: Audit of treatment of 408 cases. J Plast Reconstr Aesthet Surg. 2007;60:1030-5.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of cryotherapy and curettage for the treatment of pyogenic granuloma: A randomized trial. Br J Dermatol. 2006;154:671-5.
- [CrossRef] [PubMed] [Google Scholar]
- Cryotherapy in the treatment of pyogenic granuloma. J Eur Acad Dermatol Venereol. 2006;20:788-90.
- [CrossRef] [PubMed] [Google Scholar]
- The combined continuous-wave/ pulsed carbon dioxide laser for treatment of pyogenic granuloma. Arch Dermatol. 2002;138:33-7.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of pyogenic granuloma with the flashlamp-pumped pulsed dye laser. Pediatrics. 1997;99:368-70.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of pyogenic granuloma with a sclerosing agent. Dermatol Surg. 2001;27:521-3.
- [CrossRef] [PubMed] [Google Scholar]
- β-adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-51.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical evaluation of angiogenesis related markers in pyogenic granuloma of gingiva. Asian Pac J Cancer Prev. 2015;16:7513-6.
- [CrossRef] [PubMed] [Google Scholar]
- Stimulation of angiogenesis by Ras proteins. Biochim Biophys Acta. 2004;1654:23-37.
- [CrossRef] [PubMed] [Google Scholar]
- The use of propranolol in the treatment of infantile haemangiomas: An update on potential mechanisms of action. Br J Dermatol. 2015;172:24-32.
- [CrossRef] [PubMed] [Google Scholar]
- Becker-Shaffer's Diagnosis and Therapy of the Glaucomas. (8th ed). Mosby: United States; 2009.
- [CrossRef] [Google Scholar]
- Treatment options for cutaneous pyogenic granulomas: A review. J Plast Reconstr Aesthet Surg. 2011;64:1216-20.
- [CrossRef] [PubMed] [Google Scholar]
- Infantile hemangioma-mechanism (s) of drug action on a vascular tumor. Cold Spring Harb Perspect Med. 2011;1:a006460.
- [CrossRef] [PubMed] [Google Scholar]
- Is timolol an effective treatment for pyogenic granuloma? Int J Dermatol. 2016;55:592-5.
- [CrossRef] [PubMed] [Google Scholar]
- Ophthalmic pyogenic granulomas treated with topical timolol-clinical features of 17 cases. Ophthalmic Plast Reconstr Surg. 2018;34:579-82.
- [CrossRef] [PubMed] [Google Scholar]
- The abnormal dermis in pyogenic granuloma. Histochemical and ultrastructural observations. J Am Acad Dermatol. 1980;2:132-42.
- [CrossRef] [Google Scholar]
- Pyogenic granuloma (lobular capillary hemangioma): A clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-76.
- [CrossRef] [PubMed] [Google Scholar]






