Translate this page into:
Effectiveness and safety of levocetirizine 10 mg versus a combination of levocetirizine 5 mg and montelukast 10 mg in chronic urticaria resistant to levocetirizine 5 mg: A double-blind, randomized, controlled trial
2 Department of Pharmacology, Institute of Post Graduate Education and Research, Kolkata, West Bengal, India
3 Department of Biochemistry, Medical College and Hospital, Kolkata, West Bengal, India
4 Department of Biochemistry, Nil Ratan Sircar Medical College and Hospital, Kolkata, West Bengal, India
Correspondence Address:
Amrita Sil
Assistant Professor, Department of Pharmacology, IPGME&R 244 AJC Bose Road, Kolkata - 700 020, West Bengal
India
| How to cite this article: Sarkar TK, Sil A, Pal S, Ghosh C, Das NK. Effectiveness and safety of levocetirizine 10 mg versus a combination of levocetirizine 5 mg and montelukast 10 mg in chronic urticaria resistant to levocetirizine 5 mg: A double-blind, randomized, controlled trial. Indian J Dermatol Venereol Leprol 2017;83:561-568 |
Abstract
Background: Chronic urticaria is a vexing problem for patients and treating physicians alike. The EAACI/GA[2]LEN/EDF/WAO guidelines advocate an increased antihistamine dosage up to four times the standard, before adding leukotriene receptor antagonists. Patients are frequently intolerant of these higher dosages. We conducted this study to determine whether the addition of leukotriene receptor antagonists to the standard antihistamine dose was comparable to higher dosages of antihistamines alone, in terms of efficacy, safety and quality of life changes. We compared levocetirizine 10 mg (double dose of standard) versus a combination of levocetirizine 5 mg and montelukast 10 mg in cases of chronic urticaria not responding to single daily dose of 5 mg levocetirizine.Methods: A single-center, double-blind, randomized, active-controlled, parallel group phase IV trial (CTRI/2014/12/005261) was conducted on 120 patients of chronic urticaria of either sex not responding to 5 mg levocetirizine. Patients were randomized into receiving either levocetirizine 10 mg or levocetirizine 5 mg + montelukast 10 mg for 4 weeks. Primary outcome measures were Urticaria Activity Score (UAS) and Urticaria Total Severity Score (TSS). Routine hematological and biochemical tests and treatment-emergent adverse events were monitored for safety.
Results: Fifty-two patients on levocetirizine 10 mg group and 51 patients on levocetirizine 5 mg + montelukast 10 mg group were analyzed. UAS and TSS reduced significantly in both treatment groups and reduction of score were comparable in between the groups (P = 0.628, P = 0.824, respectively). Among adverse effects, sedation was noted significantly more (P = 0.013) in levocetirizine 10 mg group. Quality of life was significantly improved in levocetirizine 5 mg + montelukast 10 mg group (P = 0.031).
Limitations: The limitation of the study was that the follow-up period was 4 weeks.
Conclusion: EAACI/GA[2]LEN/EDF/WAO guidelines need to be more flexible in allowing usage of montelukast before escalation of anti-histamine dosage.
Introduction
Chronic urticaria, a disease characterized by itching and wheals for >6 weeks, has a significant impact on the quality of life of those affected. It is a vexing problem for the treating dermatologist as well, when first-line therapy does not succeed.[1] Being a disease of mast cell release, the various mediators of allergic response are drug targets.[2],[3] According to the EAACI/GA [2] LEN/EDF/WAO guidelines antihistamines in standard pharmacological doses are the first-line weapons, followed by an increased dosage up to fourfold if the symptoms persist after 2 weeks. The guidelines also state that leukotriene receptor antagonists are to be added only if patients do not respond to this increased dosage of antihistamines, with immunomodulators being the ultimate option for recalcitrant urticaria.[4]
Many patients do not tolerate a fourfold increased dosage of antihistamines. Although higher doses often are more effective, they also lead to increased incidence of adverse effects such as sedation, cognitive impairment, dry mouth and urinary retention.[5],[6] To obviate this problem, a leukotriene receptor antagonist could be added to a standard dose of antihistamine. Although there are trials demonstrating the role of levocetirizine,[7],[8],[9],[10] as well as leukotriene receptor antagonist (montelukast) as monotherapy,[11] and a combination of antihistamine with leukotriene receptor antagonist,[12] we were unable to find any previous double-blind, active control, parallel group trials which compared an escalated dose of levocetirizine with a combination of leukotriene receptor antagonist with standard dose (5 mg) of levocetirizine in treatment of difficult-to-treat/resistant chronic urticaria. Hence, we decided to observe through this study, whether earlier addition of leukotriene receptor antagonists could be useful for difficult-to-treat chronic urticaria patients.
In this study, we have compared the safety, effectiveness and quality of life changes of a double dose of antihistamine (levocetirizine 10 mg) with a combination of leukotriene receptor antagonist with single dose of antihistamine (levocetirizine 5 mg + montelukast 10 mg) in patients not responding to levocetirizine 5 mg alone.
Methods
The study was conducted as a single-center, double-blind, randomized (1:1) parallel group, active control trial. The recruitment period was from March 2014 to March 2015 and the total period of our study was 18 months. Patients >18 years of age of either sex presenting with history or symptoms of chronic urticaria and attending outpatient department of a teaching hospital in the eastern zone of India, were given levocetirizine 5 mg once daily, for 2 weeks. The study definition for chronic urticaria was a disease characterized by itching and wheals for >6 weeks.[1] If they failed to respond, they were screened and recruited into the trial, after providing informed consent. These (resistant) cases were defined as a persons with Urticaria Total Severity Score (TSS) ≥10 (minimum score of TSS = 10 derived by the number of wheals ≥10, size of wheals <1 cm, intensity of pruritus mild, duration of persistence <1 h, frequency of appearance daily or almost daily/week, frequency of antihistamine use daily or almost daily/week).[13] The exclusion criteria were pregnant and lactating women, patients having end-stage renal disease or those who were immunosuppressed due to drug or disease, patients with history of alcohol or substance abuse, participants working in night shifts or those likely to have a change of the usual sleep/wake cycle, patients allergic to levocetirizine, cetirizine or its parent compound hydroxyzine, montelukast and those who had a history of nonsteroidal anti-inflammatory drug intake over the last 15 days. Patients who had participated in any other clinical trial within the past 3 months, those not willing to provide written informed consent or not likely to comply with the trial protocol were also excluded from the study. The trial was approved by the Institutional Ethics Committee and has been registered in Clinical Trial Registry, India (CTRI/2014/12/005261). Computer-generated random number table was used for randomization, which was a simple randomization with 1:1 allocation to divide the patients equally into two groups. Allocation concealment was done by providing medicines in sequentially numbered, opaque sealed envelope (SNOSE).
A person unrelated to the trial packed the medicines in opaque envelopes serially according to the randomization sequence. These packs were handed over to the investigator, thus effectively blinding him to the medicine that the patient was going to receive. The tablets looked similar and were given in opaque envelopes, therefore blinding patients. Thus, double-blinding was achieved.
One treatment group was given tablet levocetirizine 10 mg while the other one was provided a combination of tablet levocetirizine 5 mg + montelukast 10 mg. Both the medications were to be consumed orally once at night after meals for 4 consecutive weeks. For levocetirizine 10 mg, the formulation marketed by Systopic Laboratories Private Limited (levosiz tablets, batch no. L 29013, manufacturing date: 10/2013, expiry date: 9/2015) was utilized. For levocetirizine 5 mg + montelukast 10 mg, the formulation marketed by Systopic Laboratories Private Limited (levosiz M tablets, batch no. LM 350913, manufacturing date: 09/2013, expiry date: 08/2015) was used. Both the medications were supplied by the Systopic Pharmaceuticals for trial purpose, and were provided free-of-cost to the patients participating in the study.
Follow-ups were carried out at weekly intervals for 4 weeks. The primary outcome measures were Urticaria Activity Score (UAS)[14] and Urticaria Total Severity Score (TSS).[13] UAS is the sum total of number and size of the wheals (0 - <10 small wheals [diameter <3 cm]; 1-10–50 small wheals or <10 large wheals [diameter >3 cm]; 2 - >50 small wheals or 10–50 large wheals; 3 - almost the whole body is covered) and the itch severity score (0 - none; 1 - mild; 2 - moderate; 3 - severe). TSS is the measure of disease activity derived from number and size of wheals, the itch severity score, duration of persistence of lesions, frequency of appearance of wheals and frequency of administration of antihistamine with each of the parameters having score of 0–3, maximum score being 18. TSS includes more parameters as compared to UAS for determining disease activity, but UAS, being simpler, is used more commonly by dermatologists. The secondary outcomes were patient's global assessment of disease activity improvement and physician's global assessment of disease activity improvement, both of which were scored on a 5-point Likert scale (0 - no improvement; 1 - mild improvement; 2 - moderate improvement; 3 - marked improvement; 4 - excellent improvement).[15] The parameters were assessed on 1st, 2nd, 3rd and 4th treatment weeks following randomization.
Safety assessment was done by seeking treatment-emergent adverse effects as reported by the patient or elicited on direct questioning by the treating physician, and routine hematological and biochemical tests. Laboratory parameters were assessed at baseline and after 4 weeks of continuous therapy with trial medication. These included hemoglobin, total leukocyte count, differential leukocyte count, platelets, erythrocyte sedimentation rate, liver function test (serum bilirubin, alkaline phosphatase, serum glutamic pyruvic transaminase, serum glutamic oxaloacetic transaminase), fasting blood sugar, urea and creatinine. Quality of life was assessed by a validated Dermatology Life Quality Index (DLQI) questionnaire in the local (Bengali) language (http://www.dermatology.org.uk/downloads/DLQI_Bengali.pdf) which comprised ten questions.[16] The aim of the questionnaire was to measure the impact of urticaria on the life of the patient. The scoring of each of the questions varied from 0 to 3, where 0 meant “not relevant” or urticaria having “no effect at all” on quality of life, 1 meant “a little” effect, 2 and 3 meant “a lot” and “very much” effect. The DLQI was calculated by by the sum of the scores of answers to all questions, resulting in a maximum of 30 and a minimum of 0. A higher score represented a greater impairment of the quality of life.
The target sample size was 49 evaluable urticaria patients in each treatment group. This was calculated to detect a difference of 2 units in TSS between groups with 90% power and 0.05 probability of type 1 error, assuming a standard deviation of 3.5 for this parameter.[10] Considering a 20% possible dropout rate, the recruitment target was approximately 60 participants per group or 120 participants overall. Continuous variables were compared by independent samples t-test (between groups) and by paired t-test (within group). Mann–Whitney U-test and Wilcoxon matched pairs signed-rank test were employed for comparison of unpaired and paired nonparametric data. For repeated measures' comparison within group, Friedman's analysis of variance was carried out followed by post hoc Dunn's test as data were nonparametric in nature. Categorical data were compared between groups by Chi-squared test or Fisher's exact test as appropriate. MedCalc version 11.6 (Mariakerke, Belgium: MedCalc Software, 2011) software was used for statistical analysis. Effectiveness analysis was done on modified intention-to-treat criteria, with all those participants who had reported for at least one post-baseline follow-up visit. Missing values were dealt with by the last observation carried forward strategy. Laboratory values were compared in patients for whom both pre- and post-treatment sets of data were available. All patients who had received at least one dose of a study drug (essentially all 120 subjects) were considered for other safety analysis.
Results
The flow of study participants is depicted in [Figure - 1]. There were no changes to the protocol made after the commencement of the study. A total of 370 patients were diagnosed clinically as chronic urticaria and given 5 mg levocetirizine for 2 weeks. Among them, 137 (37.03%) cases were resistant to conventional 5 mg daily dose of levocetirizine and were screened for the study, but 17 patients did not meet the inclusion-exclusion criteria. Hence, 120 patients were recruited, of which 103 (85.83%) patients were analyzable as per modified intention-to-treat principle – 52 cases in levocetirizine 10 mg group and 51 cases in levocetirizine 5 mg + montelukast 10 mg group. 17 patients were lost to follow-up.
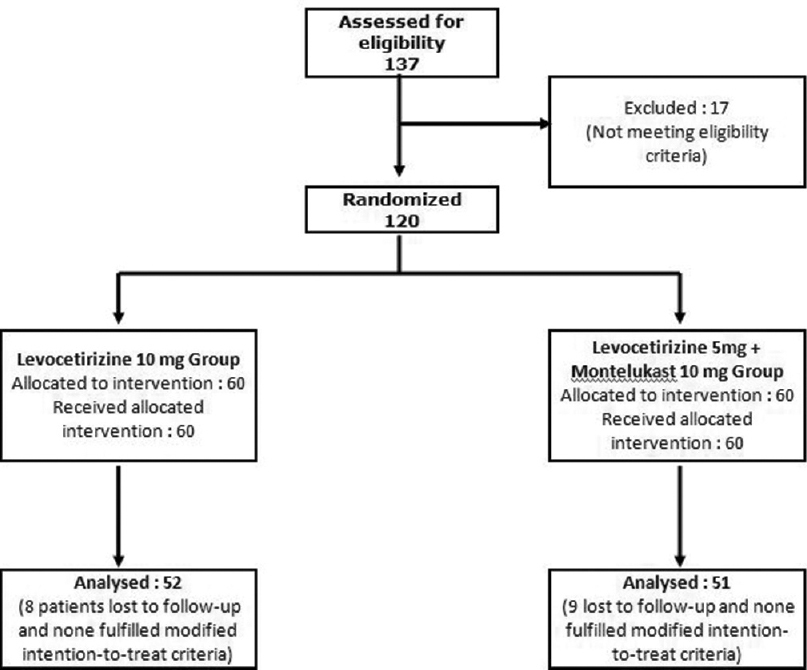 |
| Figure 1: Flow of study participants |
Most of the patients were young adult urban females in their thirties educated at or above secondary school level. Study groups were comparable with respect to age, sex, median duration of urticaria at presentation (median = 12 months) and subtypes of urticarial; 71.84% suffered from spontaneous urticaria [Table - 1].
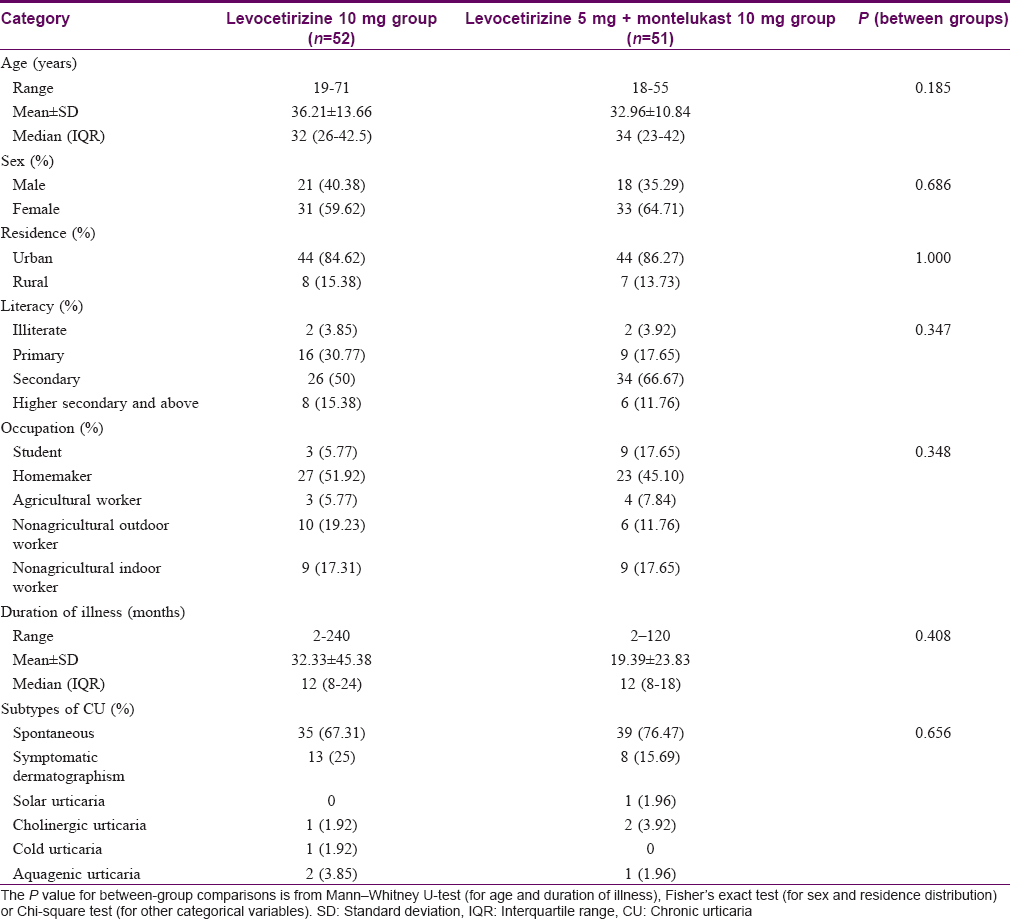
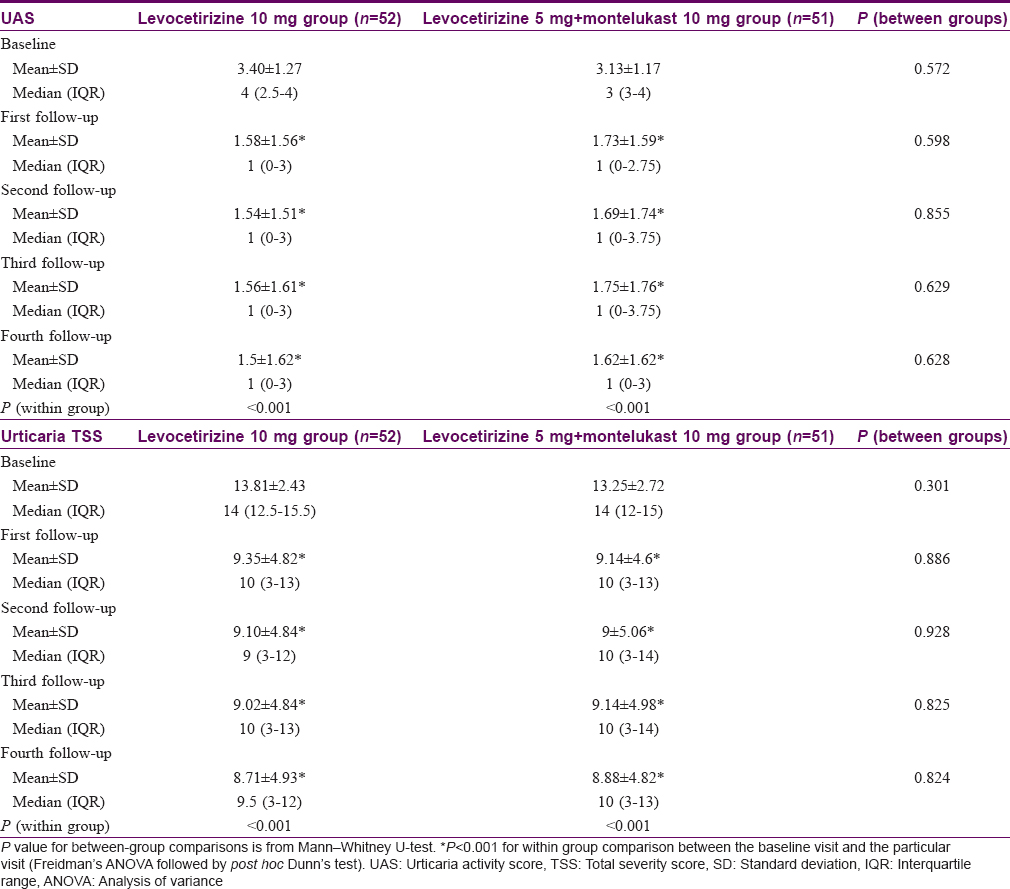
The changes in the UAS over the 4 treatment weeks are depicted in [Table - 2]. It is evident that the UAS scores in both groups are declining over this time period and this decline is statistically significant (P< 0.001) in both the treatment arms. Furthermore, when individual follow-ups were compared to baseline, it was shown to vary significantly (P< 0.001) from first follow-up onward in both treatment arms. The scores in between the treatment groups were comparable at the baseline visit and the reduction of UAS remained comparable in both groups throughout subsequent visits. There was statistically significant (P< 0.001) reduction of TSS in both the treatment groups over this period of time; furthermore, when individual follow-ups were compared to baseline, it varied significantly (P< 0.001), but like UAS, the decline of TSS was comparable in both the treatment groups at baseline and subsequent visits [Table - 2].
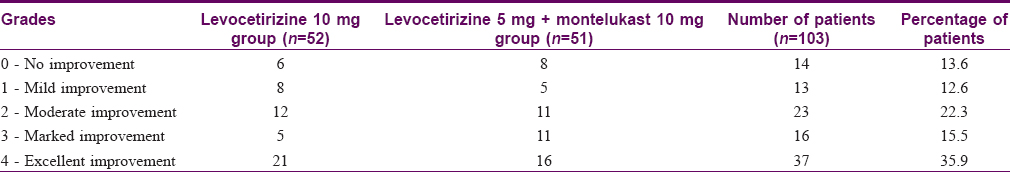
Assessment of disease severity by the physician showed that at baseline, the disease was severe (Physicians' global assessment of disease activity improvement scale value, 0–1) in both the treatment arms [Figure - 2]. However, in subsequent visits, drug treatment decreased the disease severity significantly in individual treatment groups (P< 0.001) in both the treatment arms. Individual follow-ups when compared to baseline varied significantly (P< 0.001) from first follow-up onward. Comparison in between the treatment groups showed that the decrease of disease severity was comparable in between the groups on subsequent visits. The various grades of improvement of urticaria according to Physicians' global assessment are given in [Table - 3].
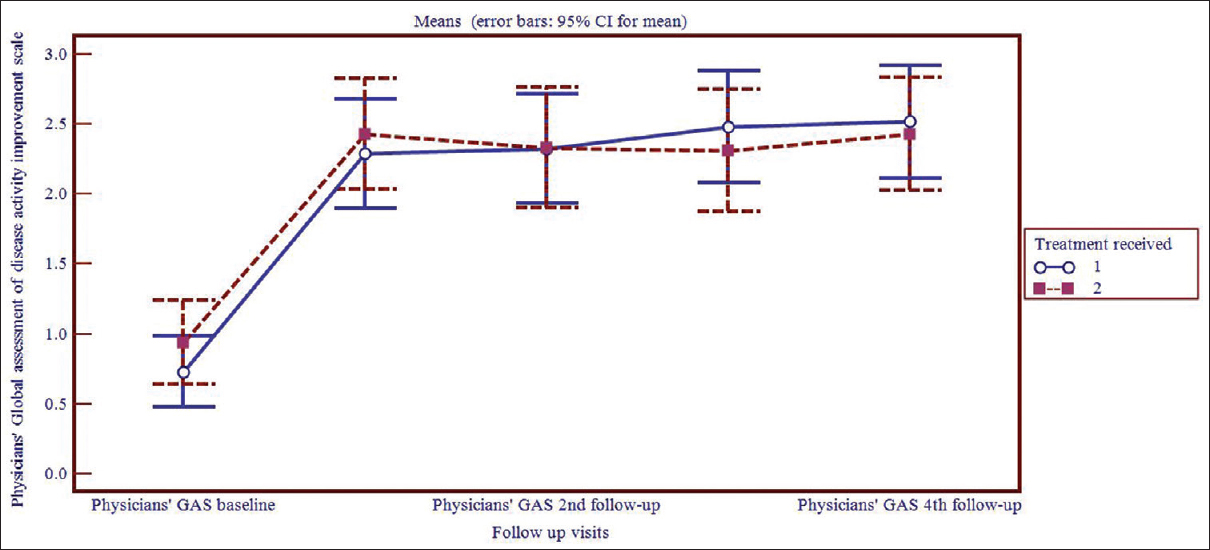 |
| Figure 2: Line diagram of physician's global assessment of disease activity improvement scale in 1 = levocetirizine 10 mg and 2 = levocetirizine 5 mg + montelukast 10 mg group |
The opinion of patients regarding their baseline disease severity did not vary in between the two treatment groups. However, as the line diagram [Figure - 3] suggests, their response to treatment was reflected in their assessment of disease severity in follow-ups, where the drugs prescribed significantly reduced their disease symptomatology in individual treatment groups (P< 0.001). Individual follow-ups when compared to baseline varied significantly (P< 0.001) from first follow-up onward in both treatment arms. Furthermore, the disease severity according to the patients' view decreased almost comparably in the levocetirizine 10 mg group and the levocetirizine 5 mg + montelukast 10 mg group during the 2nd, 3rd, 4th follow-ups.
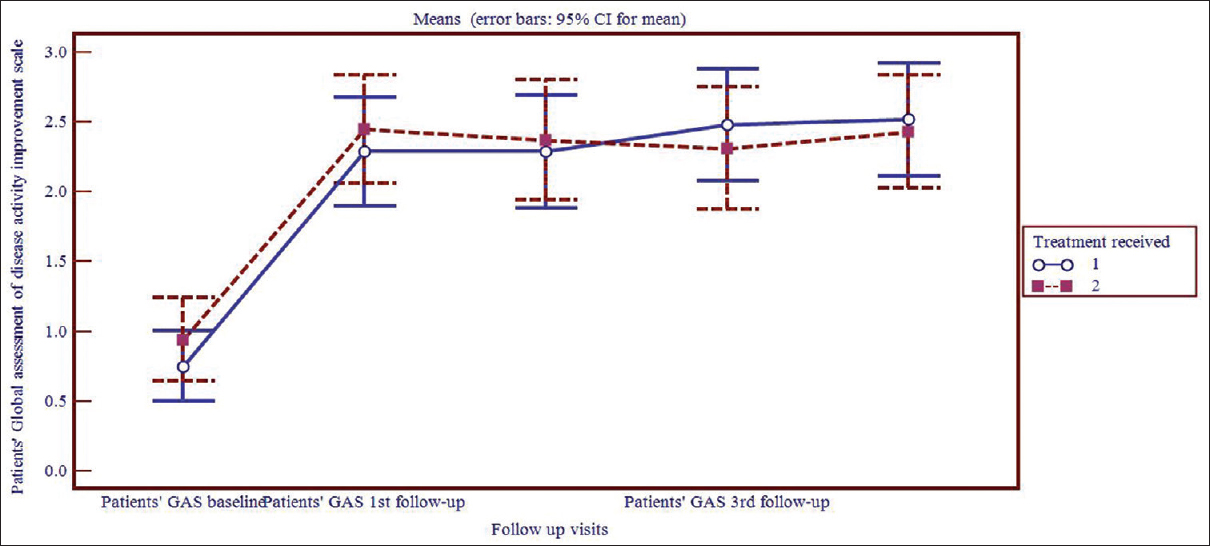 |
| Figure 3: Line diagram of patient's global assessment of disease activity improvement scale in 1 = levocetirizine 10 mg and 2 = levocetirizine 5 mg + montelukast 10 mg group |
DLQI scores decreased significantly (P< 0.0001) in both the treatment arms from baseline to 4th follow-up. However, the improvement of score was significantly better (P = 0.031) in levocetirizine 5 mg + montelukast 10 mg combination group (3.03 ± 2.29) as compared to levocetirizine 10 mg group (7.11 ± 6.31) at the end of treatment.
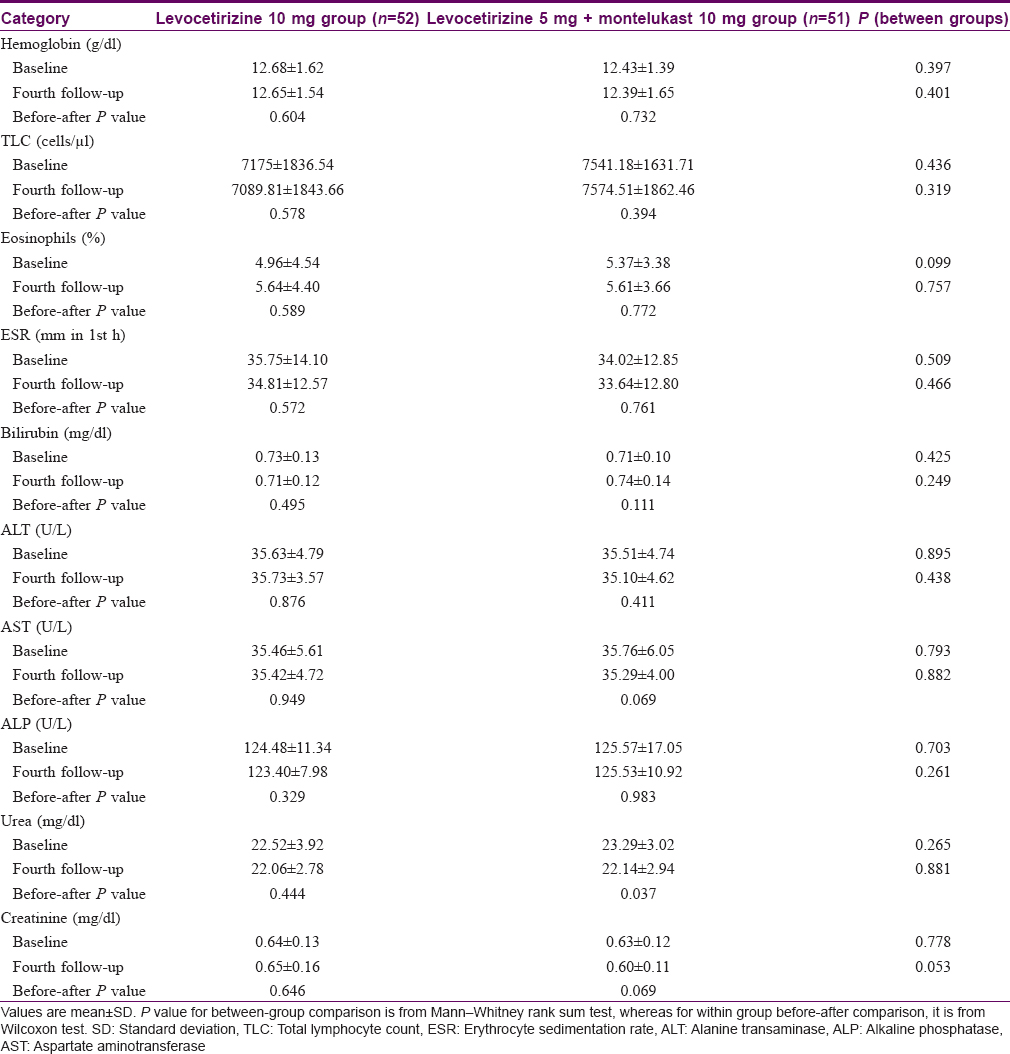
A total of 97 cases (49 in levocetirizine 10 mg group and 48 in levocetirizine 5 mg + montelukast 10 mg group) had both baseline and post-baseline (after 4 weeks) laboratory parameters available for analysis. The laboratory values were within normal limits during therapy and there were no significant changes from baseline [Table - 4]. Sedation rate was significantly higher (P = 0.013) in levocetirizine 10 mg group. Besides sedation, the other treatment-emergent adverse events noted were dizziness, fatigue, constipation, breathlessness, paresthesias of upper limbs, loss of hair, sleep disturbance and pedal edema [Table - 5]. Causality assessment was done using the World Health Organization-Uppsala Monitoring Centre scale.[17] Only “sedation” fell in the “probable” category and the rest in the “possible” category. No serious adverse events were encountered during the study period.

Discussion
The natural course of chronic urticaria is unpredictable, and thus any research on it may be intriguing for a researcher. However, the disease is exasperating for patients, as well as for the treating physician. One study had prospectively evaluated 220 patients up to 3 years and found that only 35% were free of symptoms after 1 year and among the rest, at the end of 3 years, only 47% achieved remission.[18] This highlights the fact that the therapy needs to be continued on long-term basis. For any long-term treatment regime, it is desirable that the medication should not impose significant impact on the daily activity of the patients and should be having minimum side effects. It is also desirable that the pill burden should be low as well, as it should impose minimum monetary-burden, especially in a developing country like ours, where most of the individuals are not protected by health-care insurance.
Histamine and leukotrienes are important mediators in the pathogenesis of urticaria; in principle, a combination of antihistamine and montelukast is a more rational approach in treating this condition, instead of targeting only histamine by increasing the dose of antihistamines in resistant chronic urticaria.
This study showed that most of the patients were women homemakers in their mid-thirties. Previous studies in other parts of the globe also found a greater incidence of chronic urticaria in middle-aged females similar to our study population.[19],[20]
The primary effectiveness variables, UAS [13] and TSS [14] are semiobjective methods of evaluating the activity/severity of urticaria, but TSS is superior to UAS since it incorporates the duration of persistence of symptoms and frequency of appearance of wheals. The results show that there was significant improvement in UAS and TSS in both the groups over 4 treatment weeks. Furthermore, both the scores decreased from baseline significantly (P< 0.001) during all the follow-ups in both the treatment arms, showing that both the study drugs were quite effective in relieving symptoms of urticaria. A significant reduction in both UAS and TSS was evident from the 1st week onward. Thus, urticaria, though vexing for its persistence, is rapidly managed with the present day antihistamines with higher dose or in combination with montelukast. This is the silver lining and needs to be emphasized to all patients of urticaria, particularly to those who do not respond to treatment with conventional initial standard dose of antihistamines, to uplift their morale and impart positive outlook to the disease.
Trials conducted by Erbagci [14] and Agcaoili et al.[11] demonstrated the definite role of montelukast as monotherapy, whereas Bagenstose et al. demonstrated the effective role of leukotriene receptor antagonist (zafirlukast) as add-on therapy to cetirizine in chronic urticaria.[12]
One systematic review of randomized controlled trials, showed equivocal response of leukotriene receptor antagonist (montelukast) monotherapy,[21] but combined therapy of antihistamine with leukotriene receptor antagonist seemed to be beneficial according to most of the studies.[12],[22]
The laboratory parameters showed no changes in our study, which show that both the drugs were safe in this regard. In our study, 13 (25%) patients on levocetirizine 10 mg complained of sedation whereas 3 (5.88%) patients complained of sedation in levocetirizine 5 mg + montelukast 10 mg group, suggesting lower side effect profile of montelukast. Our study showing lower incidence of sedation is corroborative with the studies conducted by Erbagci [13] and Agcaoili et al.[11] Sedation being one of the prime barriers in compliance with long-term antihistamines, less sedation with levocetirizine 5 mg + montelukast 10 mg is definitely a welcome sign and is a step forward in urticaria management. There was one patient who complained of scalp hair loss and another one who complained of pedal edema; both of them were from levocetirizine 5 mg + montelukast 10 mg group. The causality assessment showed merely a “possible association” in both cases and laboratory parameters did not suggest any specific reason for the manifestation.
Thus, a combination of levocetirizine 5 mg + montelukast 10 mg has comparable effectiveness with reduced side effects than a double dose of levocetirizine (i.e., 10 mg) which skews the benefit-risk ratio in favor of levocetirizine 5 mg + montelukast 10 mg against levocetirizine (10 mg). Needless to say with four times the dose of levocetirizine, the sedation would increase furthermore. Study participants showed very good compliance. The lack of troublesome or serious adverse events helped in this regard. High degree of compliance in the patients suffering from this chronic ailment favors the long-term therapy with either of the treatment regime.
Chronic urticaria may limit the daily activities, lead to depression and the social burden of the disease is huge. The combination of montelukast with levocetirizine by virtue of its effect on a significantly improved DLQI may help patients have a better quality of life.
For all these reasons, it is more rational to introduce a leukotriene inhibitor (e.g., montelukast) in combination with standard initial dose of antihistamine instead of increasing the dose of antihistamine alone. Hence, our study highlights that EAACI/GA [2] LEN/EDF/WAO guideline needs to be more flexible in its recommendation regarding the introduction of leukotriene inhibitors after four times the standard dose.[4] The study, being a double-blind randomized controlled trial, also provides a strong evidence (level 1b) for subsequent amendment of the existing guideline.
The limitations of the study were that the follow-up period was less, urticaria specific quality of life instrument was not used and study population included various types of urticaria and autologous serum skin test was not performed.
Conclusion
Both levocetirizine 10 mg and a combination of levocetirizine 5 mg + montelukast 10 mg can effectively treat cases of recalcitrant chronic urticaria; Sedation is much more common with higher doses of levocetirizine; quality of life is better in the montelukast arm; hence, instead of increasing the dose of antihistamines alone, the addition of leukotriene receptor inhibitors to a standard antihistamine dose can be considered in recalcitrant chronic urticaria.
Financial support and sponsorship
Institutional support was obtained for this study.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Greaves MW, Sabroe RA. ABC of allergies. Allergy and the skin. I – Urticaria. BMJ 1998;316:1147-50.
[Google Scholar]
|
| 2. |
Sabroe RA, Greaves MW. The pathogenesis of chronic idiopathic urticaria. Arch Dermatol 1997;133:1003-8.
[Google Scholar]
|
| 3. |
Kaplan AP. Urticaria and angioedema. In: Adkinson NF, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FE, editors. Middleton's Allergy: Principles and Practice. 6th ed. Philadelphia: Mosby; 2003. p. 1537-58.
[Google Scholar]
|
| 4. |
Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau A, et al. EAACI/GA(2) LEN/EDF/WAO guideline: Definition, classification and diagnosis of urticaria. Allergy 2009;64:1417-26.
[Google Scholar]
|
| 5. |
Nelson HS, Reynolds R, Mason J. Fexofenadine HCl is safe and effective for treatment of chronic idiopathic urticaria. Ann Allergy Asthma Immunol 2000;84:517-22.
[Google Scholar]
|
| 6. |
Dubertret L, Zalupca L, Cristoroulo T, Benea V, Medina I, Fantin S, et al. Once-daily rupatadine improves the symptoms of chronic idiopathic urticaria: A randomized, double-blind, placebo-controlled study. Eur J Dermatol 2007;17:223-8.
[Google Scholar]
|
| 7. |
Potter PC; Study Group. Levocetirizine is effective for symptom relief including nasal congestion in adolescent and adult (PAR) sensitized to house dust mites. Allergy 2003;58:893-9.
[Google Scholar]
|
| 8. |
Anuradha P, Maiti R, Jyothirmai J, Mujeebuddin O, Anuradha M. Loratadine versus levocetirizine in chronic idiopathic urticaria: A comparative study of efficacy and safety. Indian J Pharmacol 2010;42:12-6.
[Google Scholar]
|
| 9. |
Staevska M, Popov TA, Kralimarkova T, Lazarova C, Kraeva S, Popova D, et al. The effectiveness of levocetirizine and desloratadine in up to 4 times conventional doses in difficult-to-treat urticaria. J Allergy Clin Immunol 2010;125:676-82.
[Google Scholar]
|
| 10. |
Sil A, Tripathi SK, Chaudhuri A, Das NK, Hazra A, Bagchi C, et al. Olopatadine versus levocetirizine in chronic urticaria: An observer-blind, randomized, controlled trial of effectiveness and safety. J Dermatolog Treat 2013;24:466-72.
[Google Scholar]
|
| 11. |
Agcaoili ML, Sumpaico MW, Aleta LA, Recto MP, Abong JM. Montelukast in the treatment of chronic urticaria: A randomized double blind, placebocontrolled study. Ann Allergy Asthma Immunol 2011;107 Suppl 1.
[Google Scholar]
|
| 12. |
Bagenstose SE, Levin L, Bernstein JA. The addition of zafirlukast to cetirizine improves the treatment of chronic urticaria in patients with positive autologous serum skin test results. J Allergy Clin Immunol 2004;113:134-40.
[Google Scholar]
|
| 13. |
Bajaj AK, Saraswat A, Upadhyay A, Damisetty R, Dhar S. Autologous serum therapy in chronic urticaria: Old wine in a new bottle. Indian J Dermatol Venereol Leprol 2008;74:109-13.
[Google Scholar]
|
| 14. |
Erbagci Z. The leukotriene receptor antagonist montelukast in the treatment of chronic idiopathic urticaria: A single-blind, placebo-controlled, crossover clinical study. J Allergy Clin Immunol 2002;110:484-8.
[Google Scholar]
|
| 15. |
Bonifati C, Berardesca E. Clinical outcome measures of psoriasis. Reumatismo 2007;59 Suppl 1:64-7.
[Google Scholar]
|
| 16. |
Finlay AY. Quality of life indices. Indian J Dermatol Venereol Leprol 2004;70:143-8.
[Google Scholar]
|
| 17. |
The Use of the WHO-UMC System for Standardized Case Causality Assessment [Monograph on the Internet]. Uppsala: The Uppsala Monitoring Centre; 2005. Available from: http://www.who-umc.org/graphics/4409.pdf. [Last accessed on 2016 Mar 03].
[Google Scholar]
|
| 18. |
Torrelo A, Harto A, Ledo A. Interferon therapy for chronic urticaria. J Am Acad Dermatol 1995;32:684-5.
[Google Scholar]
|
| 19. |
Singh M, Kaur S, Kanwar AJ. Evaluation of the cause of physical urticarias. Indian J Dermatol Venerol Leprol 1990;56:109-11.
[Google Scholar]
|
| 20. |
Nettis E, Pannofino A, D'Aprile C, Ferrannini A, Tursi A. Clinical and aetiological aspects in urticaria and angio-oedema. Br J Dermatol 2003;148:501-6.
[Google Scholar]
|
| 21. |
de Silva NL, Damayanthi H, Rajapakse AC, Rodrigo C, Rajapakse S. Leukotriene receptor antagonists for chronic urticaria: A systematic review. Allergy Asthma Clin Immunol. 2014;10:24.
[Google Scholar]
|
| 22. |
Wan KS. Efficacy of leukotriene receptor antagonist with an anti-H1 receptor antagonist for treatment of chronic idiopathic urticaria. J Dermatolog Treat 2009;20:194-7.
[Google Scholar]
|
Fulltext Views
7,097
PDF downloads
1,767





