Translate this page into:
Effectiveness and safety of topical autologous platelet-rich fibrin membrane with total contact cast versus perilesional injectable autologous platelet-rich plasma therapy with total contact cast in trophic ulcer due to leprosy: A randomised controlled trial
Corresponding author: Dr. Nilay Kanti Das, Department of Dermatology, College of Medicine and Sagore Dutta Hospital, Kamarhati, Kolkata, India. drdasnilay@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mukherjee A, Das S, Roy S, Patra AC, Ghosh A, Sil A, et al. Effectiveness and safety of topical autologous platelet-rich fibrin membrane with total contact cast versus perilesional injectable autologous platelet-rich plasma therapy with total contact cast in trophic ulcer due to leprosy: A randomised controlled trial. Indian J Dermatol Venereol Leprol. 2025;91:163-71. doi: 10.25259/IJDVL_1290_2023
Abstract
Introduction
With a vision of a 90% reduction of grade 2 disability (G2D) in the Global Leprosy Strategy by 2030, the management of trophic ulcer, a common G2D, has become a priority. Autologous injectable perilesional platelet rich plasma (PRP) is first generation, whereas autologous platelet rich fibrin membrane (PRFM) is second generation platelet concentrate helping in trophic ulcer healing by providing growth factors and cytokines. PRFM requires less amount of blood (8 mL) against 20 mL in PRP.
Objectives
Evaluate the effectiveness and safety of PRFM with total contact cast versus PRP with total contact cast in leprosy trophic ulcer.
Methods
Observer-blind, non-inferiority randomised controlled trial recruited clinically diagnosed leprosy trophic ulcer with wound area measurement <40 cm2 after obtaining informed consent. Calculated sample size was 26 per group considering the percentage success in the control group (PRP) 39.29% and the experimental group (PRFM) 55.55%, 5% significance-level, 80% power, non-inferiority limit 10%, and 10% drop-out rate. Randomisation was done by computer generated random number table and allocation concealment by sequentially numbered opaque sealed envelope (SNOSE) technique. PRP was prepared with first spin 1,600 rpm for 10 minutes and second spin 4,000 rpm for 10 minutes. PRFM was prepared by centrifugation at 2,600 rpm for 3 minutes. Four treatment sessions followed by two follow-ups at 2 weekly intervals were conducted.
Results
Baseline clinico-demographic profile was similar in both groups. The surface area was significantly reduced (Friedman’s ANOVA P<0.001) in both PRP (from 422.48+657.30 sq cm to 247.84+635.96 sq cm) and PRFM (290.04+281.42 sq cm to 152.77+336.09 sq cm) with significant reduction from first FU onwards in both groups (Post-Hoc Dunn’s test P<0.001). Complete improvement was noted in 12% of PRP and 23% of PRFM (Fischer’s test P=0.465). Both groups showed improvement in DLQI.
Limitations
Short duration of treatment and follow-up (10 weeks).
Conclusion
PRFM with total contact cast is not inferior to PRP. Because of operational ease (less blood, less time), PRFM is a better alternative to PRP.
Keywords
Autologous platelet-rich-fibrin-membrane
autologous platelet-rich-plasma therapy
total-contact-cast
trophic ulcer
leprosy
Introduction
Trophic ulcers caused by leprosy have a significant impact on a patient’s life by being non-healing in nature, increasing morbidity, affecting mental well-being and diminishing the quality of life. These ulcers develop due to increased pressure in some body parts like the sole, hand, etc., resulting from lack of sensation in those areas.1 These trophic ulcers are difficult to treat and contribute to occupational and psychological morbidity leading to the stigma that is associated with the disease. With a vision of 90% reduction of grade 2 disability (G2D) in Global Leprosy Strategy by 2030, management of trophic ulcer, a common G2D, has become a priority.2,3
Studies have documented that these ulcers heal if rest for sufficient time is given,4 albeit sufficient rest is a utopian concept for those living with leprosy who mostly are economically underprivileged and are daily wage earners. Thus, a plaster cast (below the knee) can be applied to hasten healing yet maintaining ambulation. Needless to say, any method which hastens the healing, can help patients to return to their normal personal and professional life.
A few novel methods, based on platelets, have been developed for the healing of leg ulcers. Autologous perilesional injectable platelet-rich plasma (PRP) and autologous platelet-rich fibrin membrane (PRFM) are two such platelet-based therapies effective in healing of trophic ulcers in a short period of time.5 Platelets assist in wound healing by providing a few growth factors such as transforming growth factor-β, platelet-derived growth factor, insulin-like growth factor-1, etc.6
Platelet-rich plasma is a first-generation platelet concentrate.6 It is separated by a two-spin centrifugation method.7 It is a labour-intensive procedure. Bovine thrombin and calcium chloride are required as anticoagulants. Platelet-rich fibrin membrane is a second-generation platelet concentrate which is prepared by single-spin centrifugation and without any artificial biochemical modification. It is more simplified, cost-effective, and less time-consuming than PRP separation. There is a fine and flexible fibrin network formation that is suitable for cytokine enmeshment and cell migration due to the formation of equilateral junctions. There is a slow release of growth factors over a period of at least 7 days. It speeds up ulcer healing because of slow polymerisation.6
There are studies which have evaluated the role of autologous injectable PRP or PRFM in trophic ulcer healing.7,8 However, comparative studies between the two methods are very few. Thus, we aimed to compare the safety and effectiveness of PRP and PRFM in trophic ulcers due to leprosy.
Methods
The study was conducted as a unicentric, observer-blind, non-inferiority randomised controlled trial at a tertiary care rural hospital in Eastern India for a period of 2 years. Institutional Ethics Committee permission was obtained and written informed consent from each patient was taken. The trial was registered with the Clinical trial registry, India, and bears the registration number of CTRI/2021/09/036230.
Inclusion and exclusion criteria
All the adult leprosy patients who were treatment-naive for a clinically diagnosed trophic ulcer (a specific treatment for trophic ulcers not given) located on the plantar, medial, or lateral aspect of the foot and wound area (length × width) measuring <40 cm2 were included in the study. Patients having clinically diagnosed arterial or venous ulcer on leg or foot, concomitant immunodeficiency, heart disease, renal failure, malignancy, or psychiatric disorders; pregnant and lactating women; non-consenting patients; and those who had participated in any clinical trial within the last 3 months were excluded.
The calculated sample size was 23 per group considering the percentage success in the control group (platelet-rich plasma) (39.29%)7 and the experimental group (platelet-rich fibrin matrix) (55.55%)9 with 5% significance level, 80% power and non-inferiority limit (d) of 10%. Considering the 10% drop out, the target sample size was 26 in each group which translated to 52 total study participants.
Randomisation and blinding
Eligible participants after screening were randomised into either group A (receiving autologous platelet-rich fibrin membrane with total contact cast) or group B (receiving autologous injectable perilesional platelet-rich plasma with total contact cast) with an allocation ratio of 1:1 as per randomisation sequence. Randomisation was done by a computer-generated random number table by balanced unstratified randomisation using Winpepi software ETCETERA version 2.32 of WINPEPI (PEPI-for-Windows) program. Concealment of random allocation was done by sequentially numbered opaque sealed envelopes (SNOSE) technique. The card with the treatment name was inserted in an opaque sealed envelope as per randomisation and the envelope was numbered sequentially. The treating physician rendered the therapy as per the card contained in the envelope. The study was rendered observer-blind since the assessing physician was different from the treating physician and was unaware of the treatment. Observer-blinding was done to remove observer bias.
Study parameters
The primary effectiveness parameter was the reduction in ulcer surface area. Ulcer surface area was calculated with the tracing paper and cm2 graph paper at each visit. (The ulcer depth was not calculated in the present study.) The number of patients whose ulcer completely healed was taken as another effectiveness parameter. Vital signs and adverse events reported spontaneously and/or elicited by the clinician were assessed at each follow-up. Routine laboratory reports were done at baseline (haemoglobin, total leukocyte count, platelet count, total bilirubin, and creatinine) and end of active treatment in the sixth week. Quality of life in patients with trophic ulcer due to leprosy was assessed by a validated vernacular (Bengali) version of Dermatology Life Quality Index (DLQI).10
Visits and follow-ups
After the screening visit, the participants were randomised at the baseline visit into two groups. All participants were followed up at two weekly intervals for five visits post-randomisation. Active treatment with PRP or PRFM was given for the initial three visits (at 2nd, 4th, and 6th week). At the last two follow-up visits, no active treatment was given, but the outcome of treatment was observed (at the 8th and 10th week). The effectiveness parameters and adverse events were analysed at each visit and recorded in the case report form. DLQI was observed at baseline, sixth, and tenth week.
PRP preparation and inoculation
Twenty millilitres of venous blood was collected from the patient under aseptic precautions in clot vials labelled with identification data (name and age) and mixed with 2mL of sodium citrate dextrose. PRP was separated by a manual double-spin method at a temperature of 22℃–26℃. The tube was placed for first centrifugation at the rate of 1,600 revolutions per minute for 10 minutes and when plasma separated; plasma, buffy coat, and upper layers of RBCs were pipetted into another test tube. This was subjected to a second centrifugation at the rate of 4,000 revolutions per minute for 10 minutes. The upper two-thirds were separated as platelet-poor plasma (PPP – approximately 8mL) and the lower one-third was taken as platelet-rich plasma (PRP – approximately 2 mL). Calcium gluconate (0.8 mL) was added to the PPP part to form the PPP gel. PRP was injected perilesionally, after proper debridement of wound if needed, and PPP gel was applied over the lesions. A total contact cast was applied over the foot and leg for 2 weeks till the next visit.7
PRFM preparation and inoculation
Under aseptic precautions, 8 mL of blood was collected in a sterile vacutainer devoid of anticoagulant, followed by centrifugation at 2,600 revolutions per minute for 3minutes. (A vacutainer was chosen over a centrifugation tube to save cost.) A natural fibrin matrix gel was formed in the middle of the tube at the end of centrifugation; straw-coloured plasma collected to the top of the tube and red blood cells migrated to the bottom. The straw-coloured plasma was discarded and the fibrin gel was separated from the red blood cells. The fibrin gel was spread over paraffin-impregnated gauze and kept undisturbed for 20 minutes to allow forming of a membrane. Then, the fibrin membrane was applied on the ulcer base; covered with a sterile, non-adherent dressing and a total contact cast was applied over the foot and leg which was left undisturbed for 14 days.9
Statistical analysis
Normality testing was done by the D’Agostino-Pearson test. Continuous variables were compared between groups by independent samples t-test and within the group by paired t-test. Mann–Whitney U test and Wilcoxon’s matched-pairs signed rank test were employed for comparison of unpaired and paired non-parametric data. Friedman’s analysis of variance (ANOVA) was carried out with non-parametric data for within group repeated measures comparisons, followed by post-hoc Dunn’s test. Categorical data were compared between groups by chi-square test or Fisher’s exact test, as appropriate. Logistic regression analysis was done to determine the association between study variables and participants achieving complete healing of ulcers. MedCalc version 11.6 [Mariakerke, Belgium: MedCalc Software, 2011] was used for statistical analysis. Effectiveness analysis was done on a modified intention-to-treat basis with subjects reporting for at least one post-baseline follow-up visit. Missing values were dealt with by the ‘last observation carried forward’ (LOCF) strategy. Pre- and post-treatment laboratory values were compared in patients for whom both sets of data were available. For other safety analysis, all subjects who received at least one dose of a study therapy (essentially all subjects) were considered.
Results
Out of the 64 patients screened, 52 patients with leprotic trophic ulcer were recruited and randomised equally into two groups. One patient dropped out (who did not come for any of the visits). So, the data of 51 participants (group A=26, group B=25) was analysed based on the LOCF strategy. The CONSORT flow diagram of study participants is shown in Figure 1.
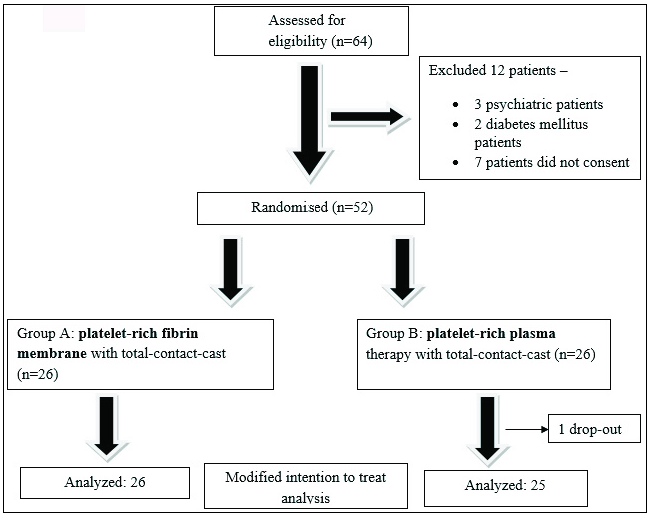
- Consolidated Standards of Reporting Trials (CONSORT) flow diagram of study participants.
There was no significant difference between the groups with respect to age (p=0.737), gender (p=1.00), education (illiterate/literate) (p=0.199), occupation (unemployed/employed) (p=0.264), and income (above poverty line/below poverty line) (p=1.00). There was male predominance in both group A (M: F=17:9) and group B (M: F=17:8) subjects [Table 1].
| Category |
Group A (Platelet-rich fibrin membrane with total contact casting) n=26 |
Group B (Platelet-rich plasma with total contact casting) n=25 |
Total N=51 |
P value (between groups) |
|---|---|---|---|---|
| Age (in years) | ||||
| Mean + SD | 45.5+ 12.47 | 44.32 + 12.46 | 44.92 + 12.35 | 0.737* |
| Median | 48 | 45 | 45 | |
| IQR | 35–55 | 40.75–54.25 | 36.25–55 | |
| Gender | ||||
| Male: Female | 17:9 | 17:8 | 34:17 | 1.000 † |
| Education | ||||
| Illiterate: Literate | 9:17 | 4:21 | 13:38 | 0.199† |
| Occupation | ||||
| Unemployed (including housewives): Employed | 14:12 | 9:16 | 23:28 | 0.264† |
| Income | ||||
| BPL: APL | 16:10 | 16:9 | 32:19 | 1.000† |
| Duration of Ulcer (months) | ||||
| Mean + SD | 51.08 + 66.05 | 24.92 + 35.41 | 38.26 + 54.38 | 0.069# |
| Median, IQR | 30, 12–48 | 12, 9–27 | 24, 11.25–36 | |
| Spectrum of leprosy | ||||
| PB, MB | 1,25 | 0,25 | 1,50 | 1.000 † |
| RFT or ongoing treatment | ||||
| RFT: Treatment ongoing | 24:2 | 22:3 | 46:5 | 0.668 † |
| Duration since beginning of MDT (months) | ||||
| Mean + SD | 84.85 + 87.97 | 72.88+ 79.46 | 78.98 + 83.28 | 0.637# |
| Median, IQR | 39, 30–114 | 48, 23.25–99 | 42, 24–105 |
SD: Standard deviation, IQR: Inter-quartile range, PRP: Platelet-rich plasma, TCC: Total contact casting, PRFM: Platelet-rich fibrin membrane, BPL: Below poverty line, APL: Above poverty line, PB: Paucibacillary, MB: Multibacillary, RFT: Relapse following treatment, MDT: Multidrug therapy; the significance of difference is tested by *unpaired t test, #Mann–Whitney test and †Fisher’s exact test
There was no significant difference between the two treatment groups according to the duration of ulcer (p=0.069), the spectrum of leprosy (p=1.00), release from treatment (RFT) (p=0.668), and duration since the beginning of MDT (0.637) [Table 1]. Overall, the most common site of ulcer was the heel (27.5%) followed by the base of the great toe (21.6%) and the great toe (19.6%) itself (Great toe meant the plantar surface of the first phalanx of the foot. Base of the great toe meant the plantar surface of the first metatarsal region of the foot).
Baseline surface area was also found to be comparable in both the treatment groups (P = 0.631, Mann–Whitney test).
Significant reduction in surface area was observed from the first follow-up onwards, in comparison with baseline in both the PRP- and PRFM-treated groups (p<0.001, Friedman ANOVA followed by post-hoc Dunn test) [Figures 2 and 3]. However, intergroup comparisons at all the subsequent follow-ups showed no statistically significant intergroup difference in surface area [Table 2].
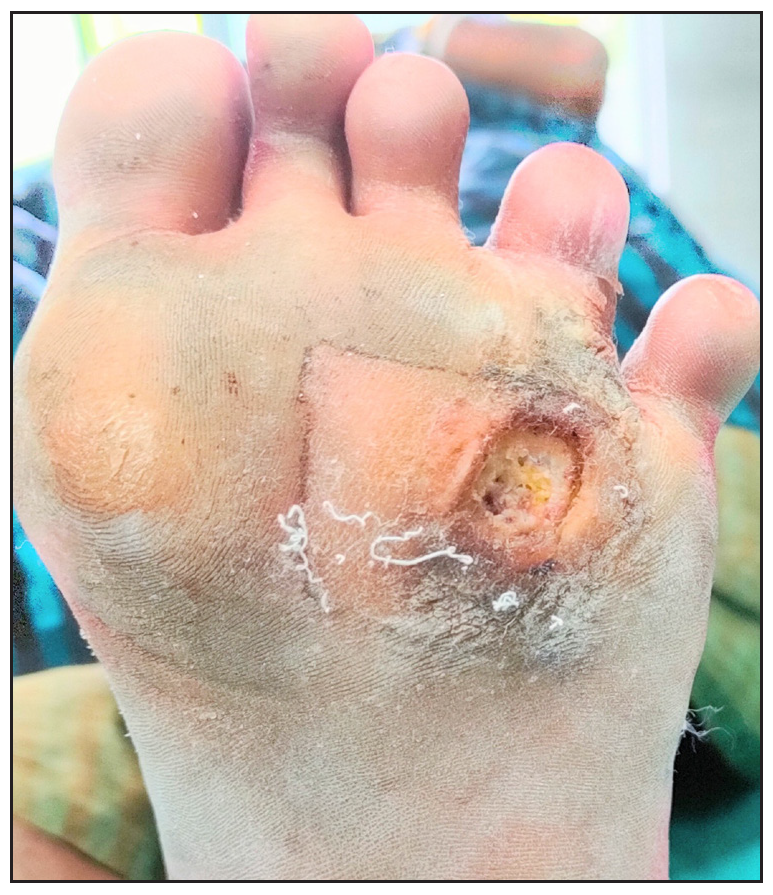
- Pre-treatment photograph with platelet-rich plasma.
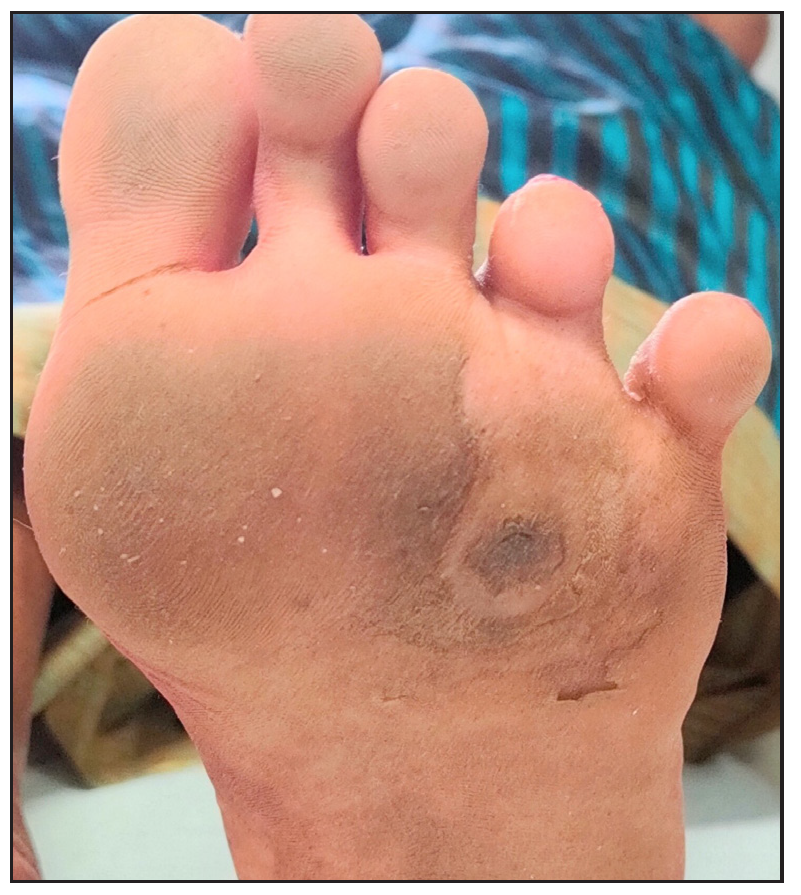
- Post-treatment photograph with platelet-rich plasma.
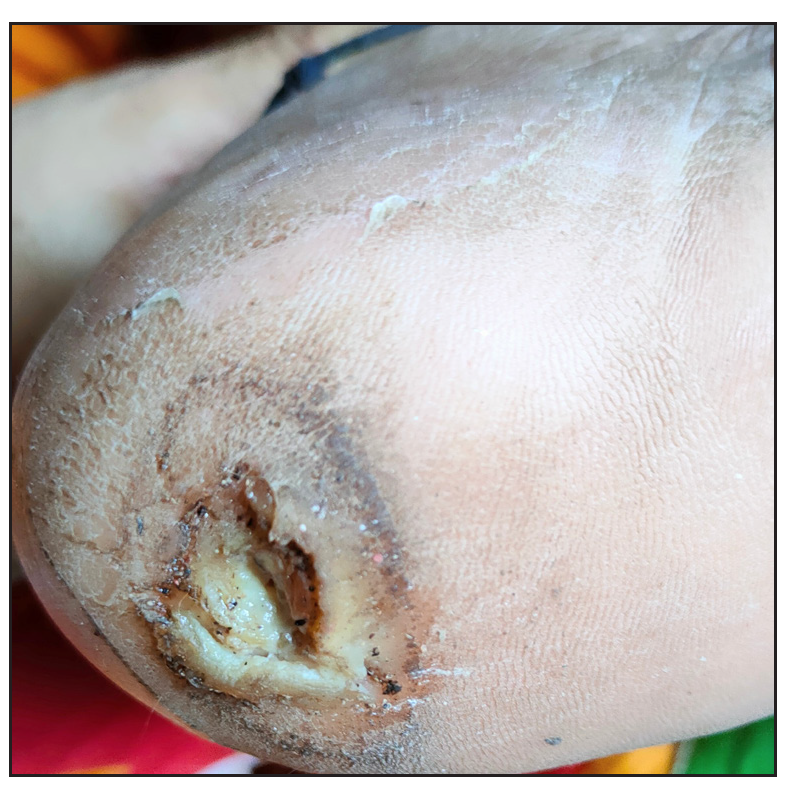
- Pre-treatment photograph with platelet-rich fibrin membrane.
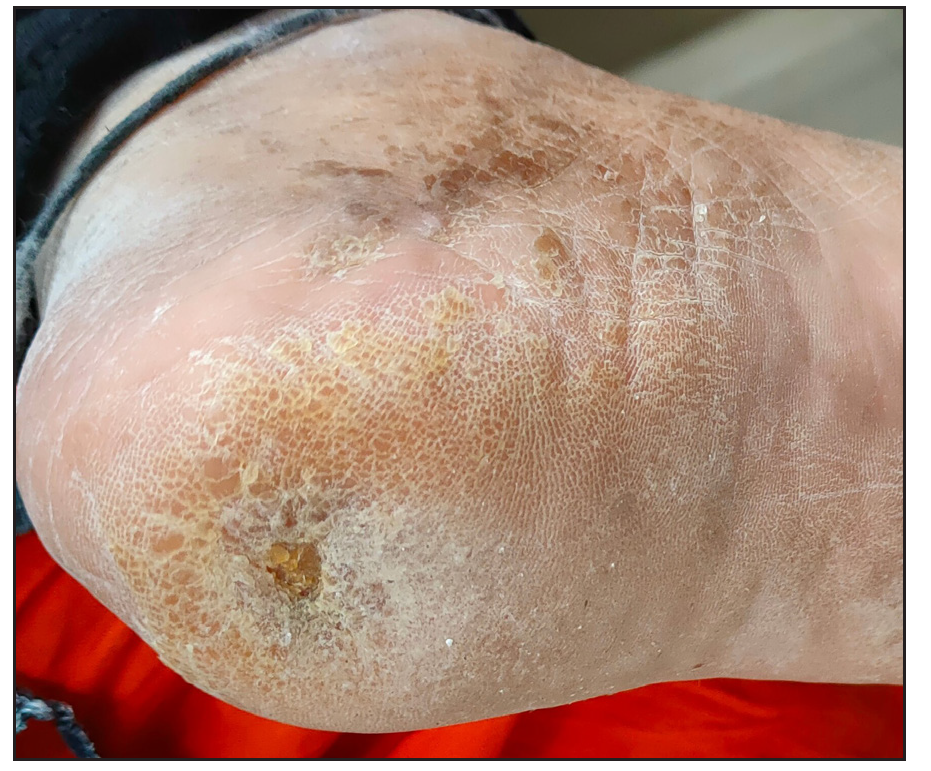
- Post-treatment photograph with platelet-rich fibrin membrane.
| Category |
Group A (Platelet-rich fibrin membrane with total contact casting) n=26 |
Group B (Platelet-rich plasma with total contact casting) n=25 |
P value (in-between groups) (Mann–Whitney test) |
|---|---|---|---|
|
Surface area baseline Mean + SD |
290.04 + 281.42 | 422.48 +657.30 | 0.631 |
|
Surface area at 1FU Mean + SD |
218.19+275.79* | 323.04+ 574.32* | 0.713 |
|
Surface area at 2FU Mean + SD |
191.15+332.17* | 258.48 +473.622* | 0.699 |
|
Surface area at 3FU Mean + SD |
163.8846+ 304.83* | 244.12+ 487.88* | 0.585 |
|
Surface area at 4FU Mean + SD |
151.69+ 335.69* | 212.56 +445.64* | 0.422 |
|
Surface area at 5FU Mean + SD |
152.77 + 336.09* | 247.84+ 635.96* | 0.637 |
|
P value (within group) (Friedman ANOVA followed by post-hoc Dunn test) |
<0.001 | <0.001 |
SD: Standard deviation, FU: Follow-up, ANOVA: Analysis of variance, PRP: Platelet-rich plasma, TCC: Total contact casting, PRFM: Platelet-rich fibrin membrane
There was no statistically significant difference in the percentage reduction of surface area between the two treatment groups at baseline and subsequent follow-ups. Intragroup change in percentage reduction of surface area was assessed by the Friedman test which shows a statistically significant difference (p<0.001), compared to baseline, in both the treatment groups. The result was further assessed by the post-hoc Dunn test to determine the follow-up or visit from which statistically significant change occurred. In both the groups, a statistically significant percentage reduction in surface area from baseline occurred from the first follow-up onwards. But, in group A, the percentage reduction at the second follow-up had no statistically significant difference from that of the first and third follow-ups; similarly, no statistically significant change was observed between the fourth and fifth follow-ups. In group B, there was no statistically significant change between the second and third follow-ups and also between the fourth and fifth follow-ups [Table 3].
| Percentage reduction of surface area |
Group A (Platelet-rich fibrin membrane with total contact casting) n=26 |
Group B (Platelet-rich plasma with total contact casting) n=25 |
P value (in-between groups) (Mann–Whitney test) |
|---|---|---|---|
| From baseline at first FU | |||
| Mean + SD | 33.76 +25.94 | 26.58 + 30.64 | 0.3263 |
| 95% CI of mean | 23.28 to 44.24 | 13.93to39.22 | |
| From baseline at second FU | |||
| Mean + SD | 47.76 + 37.77 | 48.89 + 31.39 | 1.0000 |
| 95% CI of mean | 23.76 to 71.76 | 32.17 to 65.62 | |
| From baseline at third FU | |||
| Mean + SD | 57.58 + 33.36 | 50.00 + 37.05 | 0.4915 |
| 95% CI of mean | 44.10 to 71.05 | 34.71 to 65.29 | |
| From baseline at fourth FU | |||
| Mean + SD | 67.16 + 38.44 | 58.91 + 39.60 | 0.3352 |
| 95% CI of mean | 51.63 to 82.68 | 42.56 to 75.26 | |
| From baseline at fifth FU | |||
| Mean + SD | 64.06 + 42.67 | 59.28+41.89 | |
| 95% CI of mean | 46.82 to81.29 | 41.99to76.57 | 0.5520 |
|
P value (within group) (Friedman ANOVA followed by post hoc Dunn test) |
<0.001 | <0.001 |
SD: Standard deviation, CI: Confidence interval, FU: Follow-up, ANOVA: Analysis of variance, PRP: Platelet-rich plasma, TCC: Total contact casting, PRFM: Platelet-rich fibrin membrane
The number of patients showing complete healing of the ulcer was six in group A (23.1%) and three in group B (12%). (Fischer’s test, p=0.465). Logistic regression analysis was done to find any association of variables in patients achieving complete healing of ulcers. Female gender was associated with faster healing than males (correlation coefficient −3.321). There was no significant association with age, occupation, baseline surface area, duration of ulcer, duration since the beginning of MDT, baseline haemoglobin or platelet count, and treatment with PRP or PRFM with complete healing of ulcers. [Table 4]. The different adverse events noted were painful foot swelling and nummular dermatitis in group A and painful foot swelling, callosity development, and maggot formation in group B. In group A, 24 out of 26 patients (92.31%) did not face any adverse event, whereas this figure was 22 out of 25 (88%) in group B (Fisher’s exact test: P= 0.668). The routine laboratory reports done at baseline and end of active treatment were within normal limits in both the treatment arms. The painful foot swelling was because of cellulitis and was treated successfully with antibiotics (amoxicillin and clavulanic acid 625mg three times daily for 7 days) in all the cases.
| Variable | Coefficient* | Standard error | P value |
|---|---|---|---|
| Age | 0.029 | 0.043 | 0.497 |
| Gender | −3.321 | 1.578 | 0.035 |
| Occupation | −0.005 | 0.534 | 0.993 |
| Baseline surface area of ulcer | 0.005 | 0.005 | 0.374 |
| Duration of ulcer (months) | 0.006 | 0.013 | 0.639 |
| Duration since start of MDT (months) | −0.002 | 0.012 | 0.886 |
| Baseline haemoglobin | −0.456 | 0.329 | 0.166 |
| Baseline platelet count | 0.000005 | 0.000007 | 0.420 |
| Treatment with PRP/PRFM | −0.911 | 1.062 | 0.391 |
MDT: Multi drug therapy
There was a significant decline in DLQI score from baseline at the sixth week (third follow-up visit) and tenth week (fifth follow-up visit) in both the treatment groups (Friedman test followed by post-hoc Dunn test) [Figure 4].
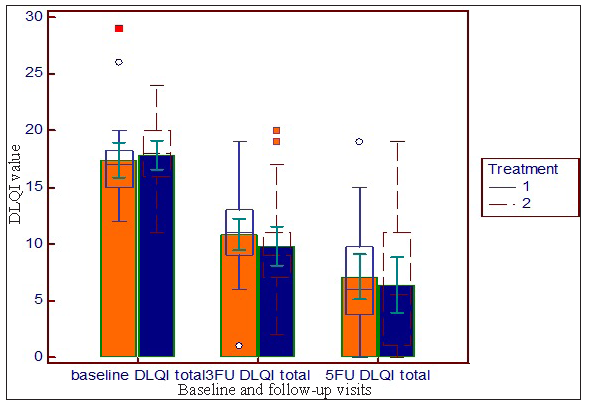
- Reduction in DLQI from baseline at sixth week baseline and follow-up visits (third follow-up visit) and tenth week (fifth follow-up visit) in both the groups (treatment 1= platelet-rich plasma, treatment 2=PRFM).
Discussion
Trophic ulcer is one of the common disabilities in leprosy. Plantar ulcers make the leprosy patients prone to stigmatisation which arises from both the self and the society. Leprosy patients with plantar ulcer need medical, social, psychological and vocational rehabilitation. So, treatment of plantar ulcers in leprosy with minimum resources is necessary for both the leprosy patients and the society.
A few novel methods have been developed for healing of trophic leg ulcers. Two of those are platelet-rich plasma therapy and PRFM. Application of total contact cast after these procedures hastens the wound healing. In this study, we compared the two methods of PRP+TCC versus PRFM+TCC in trophic ulcers due to leprosy.
For PRP preparation, we followed the protocol used by Saha S et al.7 Some authors prepared PRP in somewhat different ways. Sarvajnamurthy S et al.11 prepared PRP by centrifugation of citrate-mixed blood at 5,000 rotations per minute for 15 minutes followed by a second spin of 2,000 rotations per minute for 5–10 minutes. Bansal S et al.6 prepared PRP centrifugation of citrate-mixed blood at 1,300 rotations per minute for 10 minutes followed by a second spin of 2,000 rotations per minute for 10 minutes. Calcium chloride (0.45 mL) (instead of 0.8 mL calcium gluconate) was used to form PPP gel by Sarvajnamurthy S et al.11 Bansal S et al.6 also mentioned the use of calcium chloride (instead of calcium gluconate) for making PPP gel.
Our method of PRFM preparation was almost similar to the method used by Jagati A et al.9 except that paraffin-impregnated gauze was used instead of collagen sheet to make it more cost-effective. Nagaraju U et al.8 collected 10 mL of blood and centrifuged it at 3,000 rotations per minute for 10 minutes. Bansal S et al.6 mentioned the same rate of centrifugation as Nagaraju U et al.8 Collagen sheet was used as a scaffold by Jagati A et al.9Gauze compression followed by a secondary dressing was used as covering material by Nagaraju U et al.8 A comparison between the resources needed in PRP and PRFM is given in Table 5.
In our study, the two treatment groups were comparable regarding the clinico-demographic factors. Most of the patients were RFT and received MDT-MB. Overall, the most common site of ulceration was the heel followed by the base of the great toe.
At baseline, the study groups had analogous surface area of the ulcer. In the subsequent follow-ups, there was no statistically significant intergroup difference in surface area. In both the treatment groups, there was statistically significant reduction of ulcer surface area at each follow-up compared to baseline [Figure 5]. In a study by Nagaraju A et al.,8 significant reduction of ulcer size was observed from the first follow-up onwards by using PRFM and Saha S et al.7 also found a significant decrease in size of ulcer by using PRP from the first follow-up onwards.
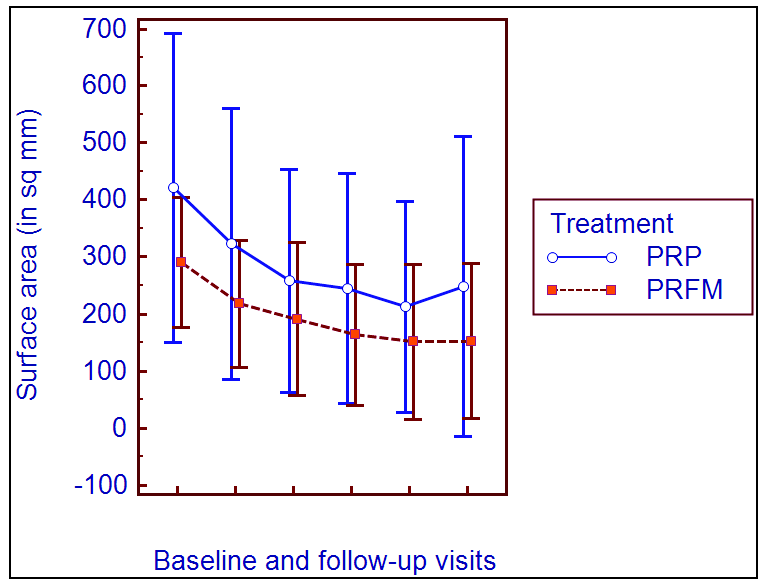
- Line diagram comparing the decrease in surface area of ulcer with platelet-rich plasma and platelet-rich fibrin membrane.
Intragroup change in percentage reduction of surface area was assessed by Friedman test which shows statistically significant difference (p<0.001), compared to baseline, in both the treatment groups. Saha S et al.7 found (91.10 + 9.65)% reduction of ulcer size by using PRP with total contactcast at the fifth follow-up compared to baseline with significant percentage reduction from first follow-up onwards. Jagati A et al.9 found 93.84% decrease in ulcer size by using PRFM over collagen sheet at the sixth follow-up. In a study by Nagaraju A et al.8 and Saha S et al.,7 significant reduction of ulcer size was observed from the first follow-up onwards.
Six out of 26 patients (23%) in group A (PRFM with total contact cast) and 3 out of 25 patients (12%) in group B (platelet-rich plasma with total contact cast) had complete healing of the ulcers. In a study by Saha S et al.,7 using PRP with total contact cast, 22 (39.29%) patients had complete resolution of ulcer, whereas Sarvajnamurthy S et al.,11 using PRP, found complete resolution in 13 (76%) patients. Jagati A et al.,9 using PRFM, found complete healing in seven (46.67%) patients, whereas Nagaraju U et al.8 observed complete resolution in all (seven) patients.
Most of the patients in both the groups did not suffer any adverse event (total= 46, 90.2%). Only five patients (9.8%) faced adverse events. In group A, 24 out of 26 patients (92.31%) did not face any adverse event, whereas this figure was 22 out of 25 (88%) in group B. The adverse events in group A (PRFM) were painful foot swelling (due to cellulitis) and nummular dermatitis; adverse events in group B (PRP) were painful foot swelling (due to cellulitis), callosity development and maggot formation. Saha S et al.7 reported some other adverse events like pain during PRP injection, iatrogenic ulcer and oedema. But, these were not noted in our study. Nagaraju U et al.8 (using PRFM) and Sarvajnamurthy S et al.11 (using PRP) did not find any adverse event.
No statistically significant intergroup difference was found at baseline, third and fifth follow-up DLQI score (Mann–Whitney test: P= 0.316 at baseline, P = 0.125 at third follow-up, P = 0.433 at fifth follow-up). DLQI was found to be significantly improved from baseline to third follow-up and fifth follow-up in both the treatment groups (Friedman test P<0.001). No other study observed a gradual change in DLQI with treatment by PRP or PRFM in leg ulcers.
The major laboratory parameters of the patients (haemoglobin, total leukocyte count, platelet count, total bilirubin and creatinine) were also checked. Most of the patients had values within normal limits. In both the treatment arms, the data were comparable between the groups. There was no statistically significant change in the laboratory parameters with treatment.
On the basis of the requirement of resources, it can be opined that platelet-rich plasma with total contact cast is more a resource and time-consuming procedure than PRFM with total contact cast. The ease of performing the technique (operational feasibility) was more with autologous platelet-rich fibrin matrix than perilesional injectable autologous platelet-rich plasma.
Table 6 shows the comparison between our study and other studies using PRP/PRFM.
| Parameters | Saha S et al.7 | Sarvajnamurthy S et al.11 | Jagati A et al.9 | Nagaraju U et al.8 | Our study |
|---|---|---|---|---|---|
| Year | 2017–18 | 2011–12 | 2019 (publication year) | 2015 | 2021–22 |
| Place | Bankura, India | Not mentioned | Ahmedabad, Wardha (India) | Not mentioned | Bankura, India |
| Type of Study | RCT | Single-arm trial | Single-arm trial | Single-arm trial | RCT |
| No. of Patients | 118 | 12 (number of ulcers=17) | 15 | 7 (number of ulcers=9) | 51 |
| PRP/PRFM | PRP (with TCC) versus TCC alone | PRP | PRFM | PRFM | Both PRP and PRFM |
| Method of Preparation | PRP: first spin 1,600 rotations per minute for 10 minutes, second spin 4,000 rotations per minute for 10 minutes | PRP: first spin 5,000 rotations per minute for 15 minutes, second spin 2,000 rotations per minute for 5–10 minutes | PRFM: 6–10 mL venous blood centrifuged at 2,200–3,000 rotations per minute for 3 min (depending upon volume of blood). The supernatant was dried over collagen sheet, kept for 20 minutes and applied over ulcer | PRFM: 10 mL venous blood centrifuged at 3,000 rotations per minute for 10 minutes and applied with a secondary dressing after gauze compression |
PRP: same as Saha S et al. PRFM: Similar to Jagati A et al. except that we used paraffin-impregnated gauze without collagen sheet to make it more cost effective |
| Duration of Treatment | 8 weeks | 6 weeks | 6 weeks | 5 weeks | 8 weeks for each group |
| Frequency of PRP/PRFM | Every 2 weeks | Every week | Every week | Every week | Every 2 weeks |
| Percentage reduction in Size |
PRP with cast: (91.1+9.65)% Total contact cast alone: (79.77+17.91)% |
(94.7+11.12)% | 93.84% | 93.52% at the end of second sitting |
PRP: (59.28+41.89)% PRFM: (64.06+42.67)% |
| Complete Reduction |
PRP with cast: 22 (39.29%) Cast alone: 11 (21.15%) |
13 (76%) | 7 (46.67%) | 9 ulcers (100%) |
PRP: 3 out of 25 (12%) PRFM: 6 out of 26 (23.1%) |
| Adverse event | Pain during injections, iatrogenic ulcers and oedema | Nil | Not mentioned | Nil | Very few–painful swelling of foot, maggot, nummular dermatitis |
RCT: Randomised controlled trial, PRP: Platelet-rich plasma, TCC: Total contact cast, PRFM: Platelet-rich fibrin membrane
Limitations
Our study was limited by the fact that the time period of active treatment was restricted to 8 weeks due to logistic reasons. We could follow-up the patient for 1 more month after active treatment but not beyond that.
Conclusion
Both PRP and PRFM were effective in reducing the surface area of the ulcer. The baseline surface area was comparable between the groups. The surface area of the ulcers reduced significantly from the first follow-up onwards in both the groups. There was a significant decline in Dermatology Life Quality Index (DLQI) score in both the treatment groups.Most of the patients in both the groups did not suffer any adverse events. We found that PRFM therapy requires less time, less patients’ blood and materials in comparison with platelet-rich plasma therapy. So, it can be concluded that PRFM may be a more cost-effective and less resource-consuming procedure than platelet-rich plasma therapy. The operational feasibility is more with the PRFM therapy.
So, PRFM is not inferior to platelet-rich plasma therapy with respect to effectiveness, safety and requirement of resources.
Ethical approval
The research/study was approved by the Institutional Review Board at Bankura Sammilani Medical College, number BSMC/Aca:-145, dated 19-01-2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Cysts, ulcers and sinuses. In: Russell R.C.G., ed. Bailey & love’s short practice of surgery (24th edition). 2004. p. :207.
- [Google Scholar]
- Global strategy for further reducing the leprosy burden and sustaining leprosy control activities (Plan period: 2006-2010). WHO-CDS-CPE-CEE-2005.53.doc. https://www.who.int/publications/i/item/WHO-CDS-CPE-CEE-2005.53. Accessed November 21, 2023.
- Global leprosy strategy 2016-20 - Accelerating towards a leprosy free world. Monitoring and evaluation guide. https://www.who.int/publications/i/item/9789290225096. Accessed November 21, 2023.
- Deformities of face, hands, feet and ulcers and their management. In: Kumar B, ed. IAL textbook of leprosy (2nd edition). New Delhi: The Health Sciences Publisher; 2016. p. :526.
- [Google Scholar]
- Platelet-rich fibrin dressings in treating nonhealing leg ulcers. J Am Acad Dermatol. 2019;80:e31-2.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich fibrin or platelet-rich plasma – Which one is better? An opinion. Indian J Dent Sci. 2017;9:S49-52.
- [Google Scholar]
- Effectiveness and safety of autologous platelet-rich plasma therapy with total contact casting versus total contact casting alone in treatment of trophic ulcer in leprosy: An observer-blind, randomized controlled trial. Indian J Dermatol Venereol Leprol. 2020;86:262-71.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous platelet-rich fibrin matrix in non-healing trophic ulcers in patients with hansen's disease. J Cutan Aesthet Surg. 2017;10:3-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Preparation of platelet-rich fibrin membrane over scaffold of collagen sheet, its advantages over compression method: A novel and simple technique. J Cutan Aesthet Surg. 2019;12:174-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Quality of life indices. Indian J Dermatol Venereol Leprol. 2004;70:143-8.
- [PubMed] [Google Scholar]
- Autologous platelet rich plasma in chronic venous ulcers: Study of 17 cases. J Cutan Aesthet Surg. 2013;6:97-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






