Translate this page into:
Effectiveness, safety and tolerability of cyclosporine versus supportive treatment in Stevens–Johnson Syndrome/Toxic Epidermal Necrolysis: A record-based study
2 Department of Pharmacology, Institute of Post Graduate Medical Education and Research, Kolkata, West Bengal, India
Corresponding Author:
Nilay Kanti Das
Department of Dermatology, Medical College and Hospital, 88 College Street, Kolkata - 700 073, West Bengal
India
drdasnilay@gmail.com
| How to cite this article: Mohanty S, Das A, Ghosh A, Sil A, Gharami RC, Bandyopadhyay D, Das NK. Effectiveness, safety and tolerability of cyclosporine versus supportive treatment in Stevens–Johnson Syndrome/Toxic Epidermal Necrolysis: A record-based study. Indian J Dermatol Venereol Leprol 2017;83:312-316 |
Abstract
Background: Toxic epidermal necrolysis and Stevens–Johnson syndrome comprise life-threatening, drug-induced mucocutaneous disease spectrum. Interest in cyclosporine, a calcineurin inhibitor that can block the function of T-cells, has increased with the discovery of the importance of granulysin in apoptosis in toxic epidermal necrolysis. In our hospital, cyclosporine is given to Stevens–Johnson syndrome/toxic epidermal necrolysis patients as an adjunctive therapy.Aims: This study is an observational, record-based study comparing the effectiveness and safety of patients receiving cyclosporine versus only supportive therapy.
Methodology: Medical records as bed-head tickets and laboratory investigation reports of Stevens–Johnson syndrome/toxic epidermal necrolysis patients admitted in the hospital over a period of 1 year were collected. Data regarding clinico-demographic profile, suspected drug causing Stevens–Johnson's syndrome/toxic epidermal necrolysis, SCORTEN, body surface area involved, treatment received and outcome were obtained.
Results: Twenty-eight patients were analyzed. Nineteen belonged to the cyclosporine group (supportive treatment + cyclosporine), nine to supportive treatment only group. Among the suspected drugs, antiepileptics formed the major group (28.6%). Five patients in the supportive only group and one in the cyclosporine group died. Time for stabilization and reepithelialization and duration of recovery were significantly lower in the cyclosporine group (P < 0.001, P= 0.007, P= 0.01, respectively). The standardized mortality ratio was 0.32 in cyclosporine group which is nearly 3.3 times lower than the only supportive treatment.
Limitations: As it was a record-based study, certain confounding factors (serum blood urea nitrogen) could not be adjusted.
Conclusion: Cyclosporine (5 mg/kg/day) for 10 days from onset of Stevens–Johnson syndrome/toxic epidermal necrolysis may decrease the risk of dying, may provide faster healing of lesions and might lead to early discharge from hospital.
Introduction
Toxic epidermal necrolysis (TEN) and Stevens–Johnson syndrome (SJS) comprise life-threatening, typically drug-induced mucocutaneous disease spectrum. The mortality rate of SJS varies between 1% and 5%, whereas TEN ranges from 25% to 30%.[1] The pathogenic mechanism involves antigenic moiety/metabolite, peptide-induced T-cell activation, leading to keratinocyte apoptosis through soluble Fas ligand, perforin/granzyme B, tumor necrosis factor-alpha and nitric oxide. Recent studies have implicated granulysin in toxic epidermal necrolysis apoptosis and have suggested that it may be the pivotal mediator of keratinocyte death.[2] The mainstay of treatment for TEN involves discontinuation of the offending drug and supportive therapy. Intravenous immunoglobulin, corticosteroids, plasmapheresis, tumor necrosis factor-alpha inhibitors, granulocyte colony-stimulating factor, N-acetylcysteine and cyclosporine have been used in therapy for patients with TEN with mixed results. Current literature does not convincingly support the use of any adjuvant systemic therapy since evidence in lacking.[2]
The primary mechanism for keratinocyte death in SJS/TEN is apoptosis. Cytotoxic T-cells are activated by an inciting drug which releases granulysin. Granulysin scissors through the membranes of target cells causing ionic instability leading to mitochondrial damage and cell apoptosis.[3] Interest in cyclosporine, a calcineurin inhibitor that can block the function of T cells, has increased with the discovery of the importance of granulysin in the apoptosis in TEN.[2] In our hospital setting, cyclosporine is given to SJS-TEN patients as an adjunctive therapy. This study is an observational, retrospective record-based study comparing the effectiveness, safety and tolerability of patients receiving cyclosporine versus supportive therapy.
Methodology
This institution-based, retrospective record-based study was conducted in the dermatology indoor setting of a tertiary care hospital, Medical College, Kolkata. Clearance from the Institutional Ethics Committee was obtained before starting the study. Medical records as bed-head tickets and laboratory investigation reports of SJS/TEN patients admitted in the hospital over a period of 1 year (between July 2014 and June 2015) were collected. Patients of either sex and of all age groups clinically diagnosed with SJS/SJS-TEN overlap/TEN, irrespective of Score of Toxic Epidermal Necrolysis (SCORTEN) value were included in the study. Those patients who were found to have received prior treatment with immunosuppressives during the course of the disease or improperly recorded bed-head tickets were excluded from the study.
Data regarding clinico-demographic profile of patient, suspected drug causing SJS-TEN, SCORTEN [4] (score to assess the severity and predict mortality in TEN patients), body surface area involved, fluid required (according to “rule of nine”), treatment received and outcome were obtained in a standard case report form.[4]
The effectiveness variables obtained were time taken for stabilization, time taken for complete reepithelialization and mortality. Stabilization of disease was defined when new lesions stopped appearing and there was no increase in the size of existing lesion. Reepithelization was defined as complete healing of the skin without any erosion/denudation. The actual death rates were compared to the predicted rates by standardized mortality ratio (SMR) analysis (sum of observed deaths/sum of expected deaths). The predicted death rate for a particular value of SCORTEN was obtained from the study of Bastuji-Garin et al.[4] SCORTEN allots one point for each of seven variables: (1) age >40 years; (2) heart rate >120 beats/min; (3) presence of comorbid malignancy; (4) occurrence of epidermal detachment >10% of body surface area on day 1; (5) blood urea nitrogen >28 mg/dL; (6) glucose >252 mg/dL and (7) bicarbonate <20 mEq/L. Mortality increases progressively from 3.2% for a patient with 0–1 point to 35.3% for a patient with three points and up to 90.0% for those with ≥5 points.[4]
The safety and tolerability parameters obtained from the medical records were adverse events documented in the bead-head ticket and routine investigations performed on a weekly basis (complete hemogram, fasting blood sugar, liver function tests, serum urea, creatinine, serum electrolytes and electrocardiogram).
MedCalc version 10.2 (Mariakerke, Belgium: MedCalc Software, 2011) was used for statistical analysis and P≤ 0.05 was considered statistically significant. The parametric data were analyzed using unpaired t-test and nonparametric data by Chi-square test as applicable. Statistical analysis to compare the mean time for stabilization, reepithelialization and duration of survival between the two groups was done using Mann–Whitney test. Survival analysis was done using log-rank test and Kaplan–Meier plots were used to plot the data graphically.
Results
It was found that all the patients received treatment according to hospital protocol. They either received cyclosporine in the dose of 5 mg/kg/day in three divided doses for 10 days along with supportive therapy or those patients who were not considered to be candidates for cyclosporine therapy, namely, hypertensive, raised serum creatinine or potassium, immunocompromised or having malignancy were given only supportive treatment. Supportive therapy focused on the maintenance and reconstitution of the barrier function of the skin, fluid balance, prevention of ocular damage, careful monitoring for and treatment of infection, high-calorie diet, banana leaf bedding and injectable antibiotics as treatment of sepsis if required (with prior skin test).
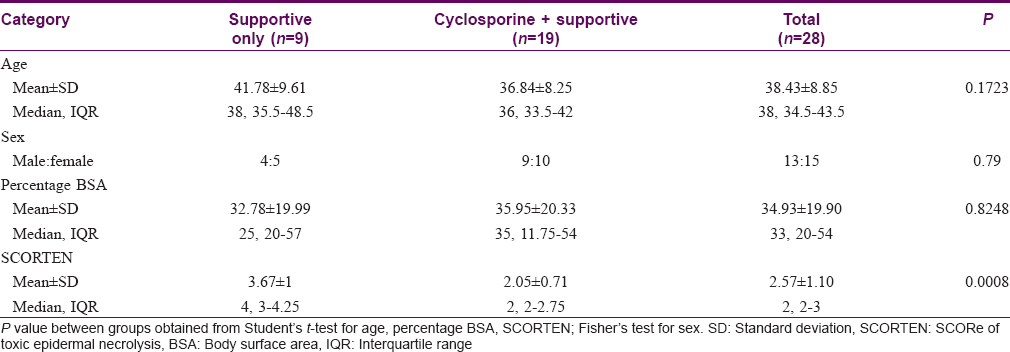
Twenty-eight patients were analyzed, of which 19 belonged to the cyclosporine group (supportive treatment along with cyclosporine) and the rest 9 to supportive treatment only group. Five patients did not fulfill the inclusion criteria. Patients were mostly middle-aged (38.43 ± 8.85 years). There was near equal distribution of either sex in our study population (male:female = 13:15). Of the patients, 6 suffered from SJS, 7 from SJS-TEN overlap and 15 from TEN. The distribution of disease in either groups was comparable (P = 0.653). The patients were admitted to the hospital at a mean of 3.08 ± 1.25 days after onset of SJS/TEN. Three patients suffered from corneal ulcer, 1 patient from synechiae of angle of mouth and one patient from symblepharon during the course of the disease. The percentage of body surface area (% body surface area) involved was an average of 34.93 ± 19.90 with no statistically significant difference between the two study groups (P = 0.825). However, the SCORTEN was significantly higher in the patients who received only supportive therapy (P < 0.001) [Table - 1].
Among the suspected drugs, antiepileptics formed the major group giving rise to SJS-TEN (28.6%) followed by ayurvedic medicines, antibiotics (14.3% each), nonsteroidal anti-inflammatory drugs (NSAIDs) (10.7%), allopurinol (7.1%), atorvastatin, thalidomide, nevirapine (3.6% each) and unidentified in 14.29% cases.
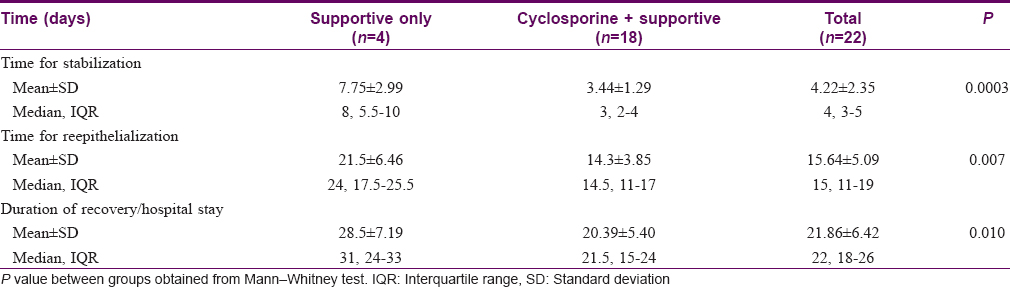
Five patients in the supportive treatment only group and one in the cyclosporine group succumbed to illness. There was a significantly higher trend of surviving in the cyclosporine group (P = 0.001) and the hazards ratio was 21.72 (95% confidence interval 3.392–139.001) [Figure - 1]. In the patients who survived, the time for stabilization and reepithelialization was significantly lower in the cyclosporine group compared to the only supportive therapy arm (P < 0.001 and P= 0.007, respectively). The mean duration of recovery was 20.39 ± 5.40 days and was significantly faster in the cyclosporine group (P = 0.01) [Table - 2].
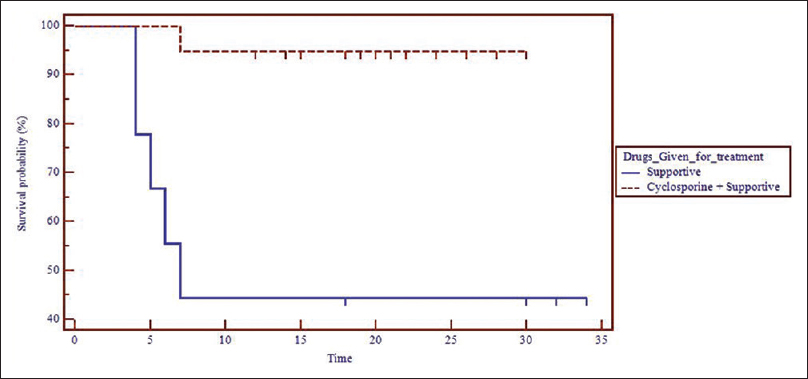 |
| Figure 1: Kaplan– Meier survival curve showing the survival of Stevens– Johnson syndrome/toxic epidermal necrolysis patients in the two study groups |
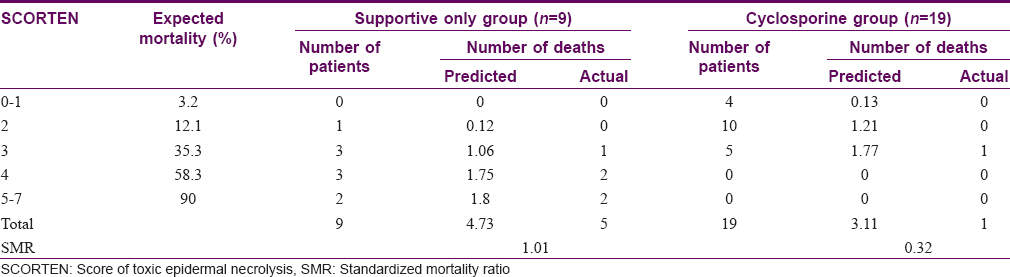
According to the SCORTEN system, the total predicted mortality in the supportive treatment only group was 4.73, whereas the actual deaths were higher (n = 5). Thus, the SMR analysis showed a value of 1.06. In the cyclosporine arm, the actual mortality (n = 1) was lower than the predicted mortality of 3.11. The SMR analysis revealed a value of 0.32 in this group which is nearly 3.3 times lower than the only supportive treatment group. The relative risk of mortality in the supportive therapy group is 2.13 times more (95% confidence interval 1.02–4.46) than those patients who received cyclosporine [Table - 3].
In the cyclosporine group, adverse events such as headache (three patients), nausea (three patients) and hypertension (two patients) were reported. All these patients were managed medically and none of these patients were withdrawn from treatment.
Discussion
Clear guidelines for the treatment of Stevens–Johnson syndrome and toxic epidermal necrolysis are lacking due to its infrequency and ethical issues of large controlled studies. Our hospital protocol abstains from the use of corticosteroids and intravenous immunoglobulin (IVIG) post the EuroSCAR study which was the largest retrospective analysis of 281 SJS/TEN patients and showed that there was no significant benefit from the use of IVIG or corticosteroids compared to supportive treatment alone.[5],[6] Furthermore, corticosteroids prolong hospital stay and make the patient more prone to complications.[7],[8]
Cyclosporine, by its novel mechanism of action and evidence from various studies, has been advocated in our hospital to treat SJS/TEN.[9],[10],[11] A Phase II open-labelled trial of cyclosporine in SJS/TEN showed that death rate and progression of detachment was lower than expected SCORTEN analysis.[10] A case series of four patients in 2011 showed a rapid improvement on administering cyclosporine.[11]
In a study by Singh et al., comparing cyclosporine-treated patients to a retrospective cohort of corticosteroid group found a significant decrease in the mean duration of reepithelialization (P = 0.01), stabilization, mean hospital stay (P = 0.03) and no mortality in the cyclosporine-treated group.[12] Our study has similar findings with the exception that there was a singular death in cyclosporine-treated arm. The death was probably due to higher body surface involvement (64%) with associated mucosal involvement of eye, mouth and genitalia with a larger lag period between onset of disease and hospital admission (5 days).
A retrospective study by Kirchhof et al. comparing IVIG and cyclosporine showed a relative mortality benefit to the use of cyclosporine in the treatment of SJS/TEN with a SMR of 0.43, over the use of IVIg with a SMR of 1.43.[13] SMR with cyclosporine in our study was 0.32 compared to supportive treatment only (SMR = 1.01) which was less than the Kirchhof study. Thus, cyclosporine has a definite mortality benefit compared to supportive treatment only.
Our study carried out over a period of 1 year recorded that antiepileptics were highest in causing SJS/TEN followed by ayurvedic medicines and antibiotics. Thus, indiscriminate and over usage of such drugs should be controlled.
The time required for stabilization, reepithelialization and hospital stay was significantly less in the cyclosporine group compared to the only supportive arm. Thus, morbidity issues and recovery time fared better which is always a welcome sign since greater duration of hospital stay incurs not only more risk to nosocomial infections but also more out-of-pocket expenses.
The actual mortality in the supportive treatment group was slightly more (5.7%) compared to the predicted mortality. However, the actual mortality in the cyclosporine group was 67.9% less than the predicted mortality suggesting the effectiveness of the drug in decreasing mortality. The relative risk of dying with only supportive treatment compared to cyclosporine therapy was twice higher with a higher trend of surviving in the cyclosporine group. The hazard of dying in the cyclosporine group was 21 times more than the supportive treatment only group.
Kaplan–Maier plot shows the survival trend in the treatment groups. The vertical gap in between the two curves shows that at a particular time point, the cyclosporine group has a greater fraction of subjects are surviving than the group receiving only supportive therapy.
The study was limited by the fact that the patients having higher blood urea nitrogen could not be given cyclosporine leading to higher SCORTEN value of the patients treated by only supportive therapy. Since this was an observational study, this confounding variable could not be adjusted.
Conclusion
From this study, we can decipher that usage of cyclosporine has encouraging results regards to morbidity and mortality. Cyclosporine (5 mg/kg/day) for 10 days from onset of SJS/TEN may decrease the risk of dying, may provide faster healing of lesions and might favor early discharge from hospital though the results of this observational study need to be confirmed by a randomized controlled trial after obtaining Institutional Ethics Committee approval. Ethical issues regarding randomized clinical trials in SJS/TEN have made such observational studies valuable in providing data to treat this dreaded drug-induced disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. | Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis 2010;5:39. [Google Scholar] |
| 2. | Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol 2013;69:187.e1-16. [Google Scholar] |
| 3. | Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol 2013;69:173.e1-13. [Google Scholar] |
| 4. | Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: A severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol 2000;115:149-53. [Google Scholar] |
| 5. | Schneck J, Fagot JP, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M. Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: A retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol 2008;58:33-40. [Google Scholar] |
| 6. | Del Pozzo-Magana BR, Lazo-Langner A, Carleton B, Castro-Pastrana LI, Rieder MJ. A systematic review of treatment of drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in children. J Popul Ther Clin Pharmacol 2011;18:e121-33. [Google Scholar] |
| 7. | Chave TA, Mortimer NJ, Sladden MJ, Hall AP, Hutchinson PE. Toxic epidermal necrolysis: Current evidence, practical management and future directions. Br J Dermatol 2005;153:241-53. [Google Scholar] |
| 8. | Gerdts B, Vloemans AF, Kreis RW. Toxic epidermal necrolysis: 15 years' experience in a Dutch burns centre. J Eur Acad Dermatol Venereol 2007;21:781-8. [Google Scholar] |
| 9. | Valeyrie-Allanore L, Wolkenstein P, Brochard L, Ortonne N, Maître B, Revuz J, et al. Open trial of ciclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol 2010;163:847-53. [Google Scholar] |
| 10. | Aré valo JM, Lorente JA, González-Herrada C, Jimé nez-Reyes J. Treatment of toxic epidermal necrolysis with cyclosporin A. J Trauma 2000;48:473-8. [Google Scholar] |
| 11. | Reese D, Henning JS, Rockers K, Ladd D, Gilson R. Cyclosporine for SJS/TEN: A case series and review of the literature. Cutis 2011;87:24-9. [Google Scholar] |
| 12. | Singh GK, Chatterjee M, Verma R. Cyclosporine in Stevens-Johnson syndrome and toxic epidermal necrolysis and retrospective comparison with systemic corticosteroid. Indian J Dermatol Venereol Leprol 2013;79:686-92. [Google Scholar] |
| 13. | Kirchhof MG, Miliszewski MA, Sikora S, Papp A, Dutz JP. Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. J Am Acad Dermatol 2014;71:941-7. [Google Scholar] |
Fulltext Views
3,894
PDF downloads
2,253





