Translate this page into:
Effects of air pollution on the skin: A review
2 Department of Environmental Toxicology Laboratory, National Institute of Pathology-ICMR, Safdarjung Hospital Campus, New Delhi, India
Correspondence Address:
Sushruta Kathuria
Department of Skin and STD, Vardhman Mahavir Medical College, Safdarjung Hospital, New Delhi - 110 029
India
| How to cite this article: Puri P, Nandar SK, Kathuria S, Ramesh V. Effects of air pollution on the skin: A review. Indian J Dermatol Venereol Leprol 2017;83:415-423 |
Abstract
The increase in air pollution over the years has had major effects on the human skin. Various air pollutants such as ultraviolet radiation, polycyclic aromatic hydrocarbons, volatile organic compounds, oxides, particulate matter, ozone and cigarette smoke affect the skin as it is the outermost barrier. Air pollutants damage the skin by inducing oxidative stress. Although human skin acts as a biological shield against pro-oxidative chemicals and physical air pollutants, prolonged or repetitive exposure to high levels of these pollutants may have profound negative effects on the skin. Exposure to ultraviolet radiation has been associated with extrinsic skin aging and skin cancers. Cigarette smoke contributes to premature aging and an increase in the incidence of psoriasis, acne and skin cancers. It is also implicated in allergic skin conditions such as atopic dermatitis and eczema. Polyaromatic hydrocarbons are associated with extrinsic skin aging, pigmentation, cancers and acneiform eruptions. Volatile organic compounds have been associated with atopic dermatitis. Given the increasing levels of air pollution and its detrimental effects on the skin, it is advisable to use strategies to decrease air pollution.Introduction
Pollution is defined as contamination of the earth's environment with materials which interfere with human health, quality of life, or the natural functioning of the ecosystem. The major types of pollution are water pollution, air pollution, noise pollution and soil pollution. The World Health Organization defines air pollution as contamination of the indoor or outdoor environment by any chemical, physical, or biological agent that modifies the natural characteristics of the atmosphere.[1] The sources of air pollution could be natural sources such as volcanic eruptions, forest fires, biological decay, pollen grains, marshes and radioactive materials or human-made sources such as thermal power plants, industries, vehicular emissions, household combustion devices, fossil fuel burning and agricultural activities. Pollutants of the major public health concern include particulate matter, carbon monoxide, ozone, nitrogen dioxide and sulfur dioxide.[1] Air pollution is responsible for a large proportion of health-related problems.[2]
Mechanism of Skin Damage by Air Pollutants
Living organisms are exposed to air pollutants which have major effects on the human skin. Air pollutants can exist as solids, liquids, gases and particulate matter. These are absorbed directly through the skin into the subcutaneous tissue or via hair follicles and sweat/sebaceous glands. Rapid urbanization and increased energy consumption worldwide have exposed the human body to increased quantities of ambient air pollution. The skin, being the largest and outermost body organ, acts as a physical, chemical and an immunological barrier against the environmental factors. Human skin is exposed not only to natural environmental factors but also to pollutants of anthropic origin.[3] Whenever a prolonged and repetitive exposure to environmental stressors exceeds the skin's normal defensive potential, there is a disturbance in the skin barrier function leading to the development of various skin diseases.[3] Major air pollutants which affect the skin are solar ultraviolet radiation, polycyclic aromatic hydrocarbons, volatile organic compounds, nitrogen oxides, particulate matter, cigarette smoke, heavy metals and arsenic.
Air pollutants exert a harmful effect on the skin by increasing oxidative stress which counters the skin's antioxidant defenses. There is a depletion of enzymatic (glutathione peroxidase, glutathione reductase, superoxide dismutase and catalase) and non-enzymatic (Vitamin E, Vitamin C and glutathione) antioxidant capacity. Free radicals and reactive oxygen species are generated that interact with the lipid-rich plasma membrane to initiate the lipid peroxidation reaction cascade. Reactive oxygen species also stimulate the release of pro-inflammatory mediators which results in the accumulation of neutrophils and other phagocytic cells that further generate free radicals, thereby resulting in a vicious cycle. Oxidative stress initiates complex biological processes resulting in genetic damage, activation of transcription factors such as activator protein 1 and nuclear factor kappa B, and signalling pathways such as extracellular signal-regulated kinases, c-Jun N-terminal kinases and p38 mitogen-activated protein kinases, involved in cell growth and differentiation and in the degradation of the connective tissue of the dermis. Air pollutants induce severe alterations of the normal functions of lipids, deoxyribonucleic acid and/or proteins in the human skin via oxidative damage, leading to extrinsic skin aging, inflammatory or allergic conditions such as contact dermatitis, atopic dermatitis, psoriasis, acne and skin cancer.[4],[6],[7],[8]
Air Quality Guidelines
The World Health Organization air quality guidelines are based on four major air pollutants, namely particulate matter, ground-level ozone, nitrogen dioxide and sulfur dioxide.[1] The World Health Organization guidelines for the various air pollutants are shown in [Table - 1]. The Revised National Ambient Air Quality Standards, 2009, are also shown in [Table - 1].[9] Another measure for air pollution is air quality index based on five major air pollutants regulated by the Clean Air Act:- ground-level ozone, particulate matter, carbon monoxide, sulfur dioxide and nitrogen dioxide. Its value lies between 1 and 500, with higher values indicating more air pollution. Air quality index value of 50 represents a good air quality with some potential to affect public health, while an air quality index value over 300 represents hazardous air quality. Real-time records of air quality index in New Delhi show that it is between 150 and 170, which is categorized as unhealthy.[10] This article focuses on the detrimental effects of air pollutants on various skin disorders.

Air Pollutants and their Role in Skin Diseases
Ultraviolet radiation
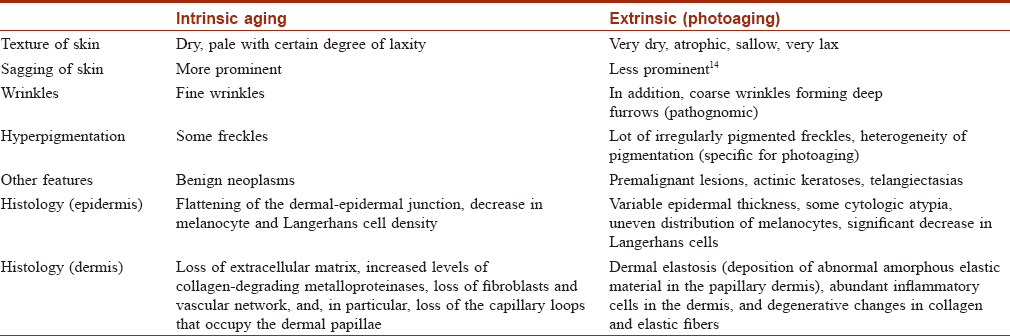
Ultraviolet radiation is a physical pollutant. The solar spectrum consists of ultraviolet A (320–400 nm), ultraviolet B (290–320 nm) and ultraviolet C (200–290 nm). More than 95% of ultraviolet A and 1–5% of ultraviolet B reach the Earth's surface, whereas most ultraviolet C is absorbed by the ozone layer and oxygen in the atmosphere.[11] The depletion of stratospheric ozone by environmental pollutants such as photochemical smog, supersonic aircraft flights and refrigerant gases increases the penetration of the shorter ultraviolet wavelengths to the ground level. The effects of ultraviolet radiation on human skin differ depending on the wavelength. Ultraviolet A exposure results in extrinsic skin aging (photoaging) characterized by coarse wrinkles, solar elastosis and pigment irregularities. Aging results from the combined action of intrinsic and extrinsic factors. The general aging process which is genetically determined and occurs in all skin by passing time is called intrinsic aging [Figure - 1] and [Figure - 2] whereas skin aging induced by environmental factors is termed as extrinsic aging. Clinical signs of extrinsic aging include solar elastosis [Figure - 3], pigment spots [Figure - 4], coarse wrinkles and telangiectasias. The differences between intrinsic and extrinsic aging are tabulated in [Table - 2].[12],[13],[14],[15]
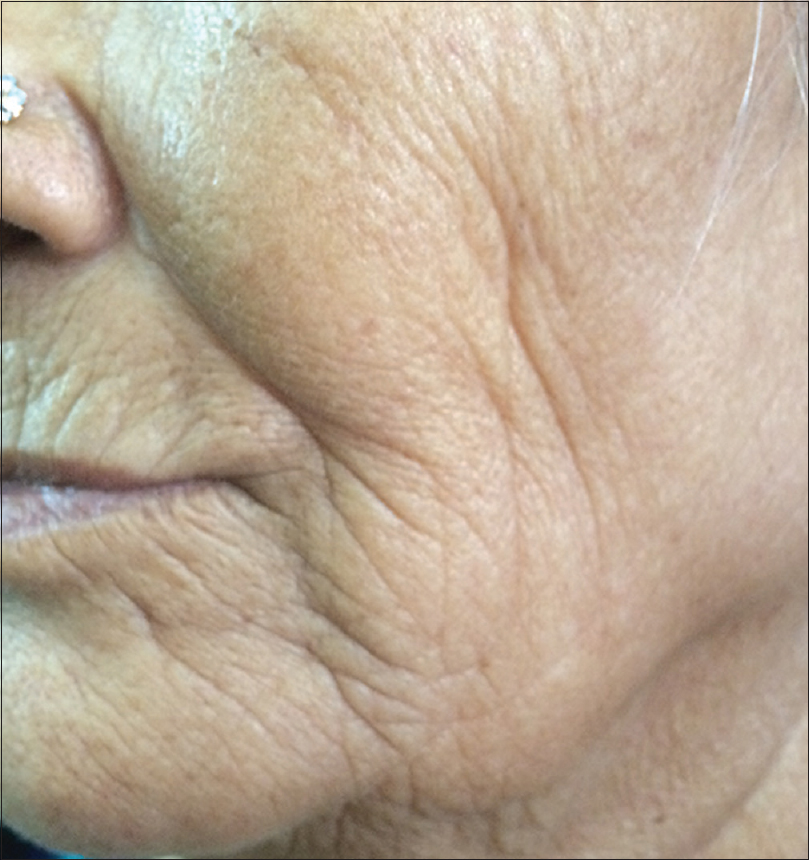 |
| Figure 1: Intrinsic aging. Sagging and lax skin with deep wrinkles in a 63-year-old woman |
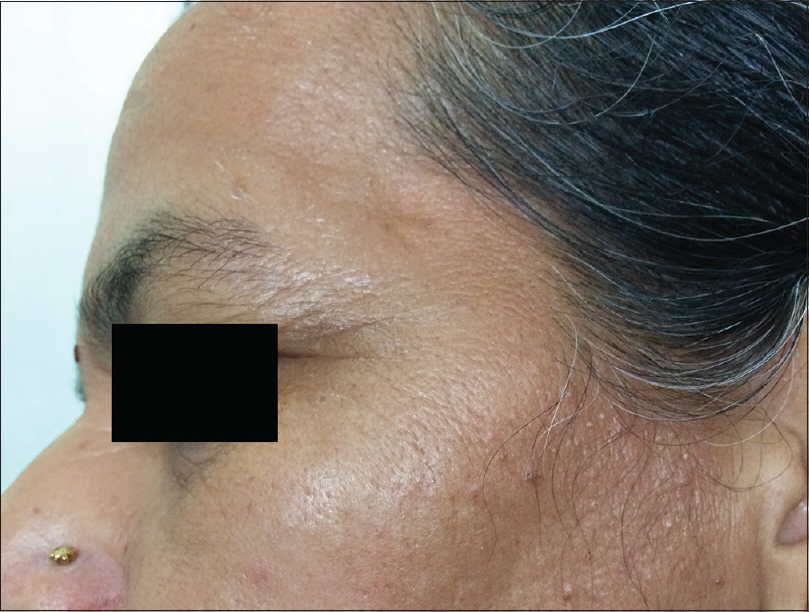 |
| Figure 2: Intrinsic aging. Fine wrinkles in a 45-year-old woman |
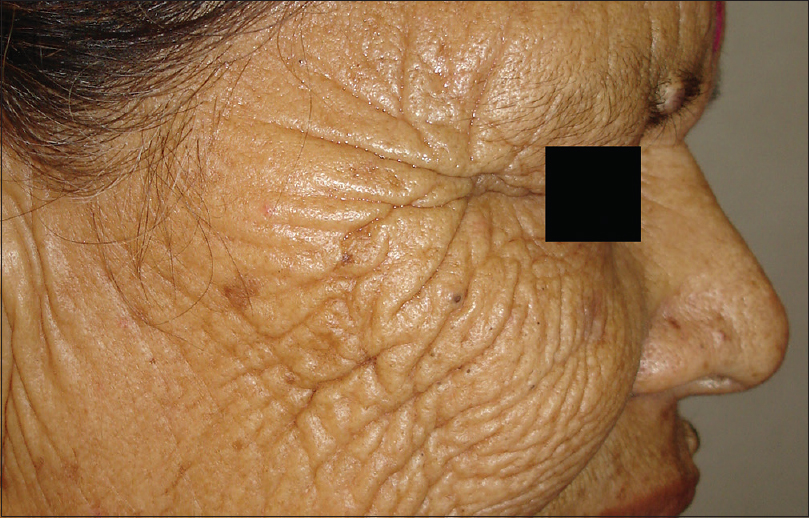 |
| Figure 3: Extrinsic aging. Solar elastosis in a 40-year-old woman living in hilly region |
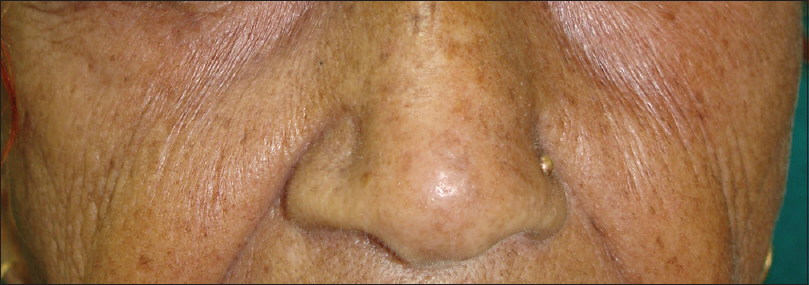 |
| Figure 4: Extrinsic aging. Freckles in a 55-year-old woman |
Photoaging is mainly caused by ultraviolet A. In an in vitro study, it was seen that ultraviolet B may also contribute to photoaging by increasing stratifin. Stratifin is a member of the 14-3-3 protein family, secreted by keratinocytes and its expression was more in skin exposed to ultraviolet B than in skin protected by ultraviolet B.[16] The mechanism of premature/extrinsic skin aging induced by ultraviolet rays is a complex process, which is triggered by various pathways, namely activation of receptors, mitochondrial damage, protein oxidation, alteration of Ca 2+ levels, telomere damage and arylhydrocarbon receptor activation. The stimulation of receptor pathway is induced by the generation of reactive oxygen species on exposure to ultraviolet radiation, smoking and air pollutants. As a result, the cell surface receptors of cytokines and growth factors in keratinocytes as well as fibroblasts are activated which leads to the intracellular stimulation of intracellular kinases, inducing the transcription of nuclear transcription factor activator protein 1 and nuclear factor kappa B. Increased activator protein 1 transcription decreases the gene expression of the major dermal collagens I and III in fibroblasts leading to a reduction in collagen synthesis. Activator protein 1 also increases the synthesis of matrix metalloproteinases in keratinocytes and fibroblasts resulting in the increased degradation of mature dermal collagen. On the other hand, nuclear factor kappa B stimulates the transcription of inflammatory cytokines resulting in the accumulation of neutrophils. The collagenases in neutrophils help in collagen degradation.
Ultraviolet B alone is responsible for sunburn. Ultraviolet A and B both have been implicated in cutaneous immunosuppression and skin cancers (photocarcinogenesis) such as malignant melanoma, basal cell carcinoma and squamous cell carcinoma.[3],[7],[17] There is a growing concern worldwide regarding the increased incidence of skin cancer. Excessive exposure to sunlight and tanning lamps is responsible for cumulative damage, which induces immunosuppression responsible for skin cancer.[18] Fair-skinned people are more susceptible than dark-skinned individuals. Australia has the world's highest rate of skin cancer which is due to high sunshine levels. Depletion of ozone increases the penetration of ultraviolet rays to the earth's surface, leading to an increase in incidence of skin cancers, and a decrease in their age of onset.[19],[20] Ultraviolet A and B damage deoxyribonucleic acid through different mechanisms. Melanocytes in the basal layer of epidermis produce the pigment melanin, which protects the neighbouring keratinocytes from ultraviolet radiation. However, prolonged exposure to sunlight can induce carcinogenesis. Ultraviolet A and B are absorbed by proteins, lipids, nuclear and mitochondrial deoxyribonucleic acid, causing a cascade of oxidative events which results in the deterioration of structure and function of cells. Ultraviolet A causes mutation of tumor suppressor p53 gene in the basal layer which has stem cells, and thus leads to carcinogenesis, which is due to deoxyribonucleic acid damage, gene mutation and immune suppression.
Ultraviolet A in combination with common environmental pollutants, such as polycyclic aromatic hydrocarbons, significantly increases visible photodamage in the skin.[21] Ultraviolet A in combination with ozone causes synergistic oxidative stress in human skin.[22] Some air pollutants (ozone, nitrogen dioxide and sulfur dioxide) and scattering particulates (clouds and soot) in the troposphere reduce the effects of shorter wavelength ultraviolet radiation, mainly ultraviolet B, and cause significant reduction in ultraviolet B irradiance in polluted urban areas. There is a reduction of more than 50% in ultraviolet radiation (mainly ultraviolet B) on days with high levels of air pollution. An inverse relationship exists between the total ozone content and ground levels of ultraviolet B radiation. Ultraviolet A, however, is not affected much in the presence of air pollutants and ozone. Therefore, the ratio of ultraviolet B/ultraviolet A is highly dependent on factors such as thickness of ozone layer and air pollution.[23]
Cigarette smoke
Cigarette smoke is a highly complex aerosol composed of thousands of chemical substances, including reactive oxygen species, carbon monoxide, reactive nitrogen species and electrophilic aldehydes.[3] Reactive oxidants and free radicals from cigarette smoke cause oxidative stress or secondary oxidative events and inhibition of antioxidant mechanisms.[24],[25],[26] Chemical substances from cigarette smoke increase transepidermal water loss, degeneration of connective tissue in the skin and upregulation of matrix metalloproteinases-1 and 3 which degrade collagen and elastic fibers.[3],[27],[28]
Smoking causes premature aging which clinically manifests as deeper periorbital wrinkling.[29],[30],[31] Premature facial skin aging in smokers, with a characteristic pattern of wrinkling and orange-purple skin discoloration, was defined as smoker's face.[32] Smoker's face typically has lines or wrinkles radiating at the right angles from the upper and lower lips or corners of the eyes, deep lines on the cheeks, or numerous shallow lines on the cheeks and lower jaw. The bony contours become prominent, and the skin is slightly pigmented gray with orange, purple and red complexion. Heavy cigarette smokers were 4.7 times more likely to have facial wrinkles than nonsmokers, independent of sun exposure, although the combination of smoking and sun exposure may have a synergistic effect.[31],[32],[33] It has been observed that wrinkling in a 40-year-old smoker resembles that of a 70-year-old nonsmoker.[34] Sometimes, large open and closed comedones with furrows (smoker's comedones) [Figure - 5] are seen in the periorbital area, similar to those seen in Favre–Racouchot syndrome. There is yellow discoloration of nails, and in persons who have stopped smoking, a sharp demarcation line may be seen between the yellow nail plate and the newly formed pink nail plate (known as Harlequin nail or quitter's nail). Other changes noticed in smokers include yellowish discoloration of the hair and beard (e.g. smoker's moustache), premature graying and loss of hair, gingival pigmentation (smoker's melanosis), leukoplakia of the tongue (smoker's tongue), oral leukoplakia [Figure - 6] and a gray-white keratinized palate with multiple red umbilicated papules that represent inflamed salivary glands (smoker's palate/nicotine stomatitis).[35] Various mechanisms have been postulated for premature aging caused by cigarette smoke. In mice models, second-hand smoke, also known as environmental tobacco smoke, involuntary smoke and passive smoke caused premature aging by increased cytoplasmic translocation of high-mobility group box 1 protein, and hence, the loss of collagen.[36] Transcription of p16INK4a has been associated with aging, and p16INK4a is a known gerontogen. In murine models, cigarette smoke and ultraviolet light have augmented the transcription of p16INK4a.[37] Cigarette smoke extract caused senescence of fibroblasts, possibly by oxidative stress injury and inhibition of antioxidant defense activity in in vitro studies.[38] Cigarette smoke-induced early growth response-1 induces the expression of cysteine-rich 61 in human skin dermal fibroblasts which may be the cause of premature aging.[39]
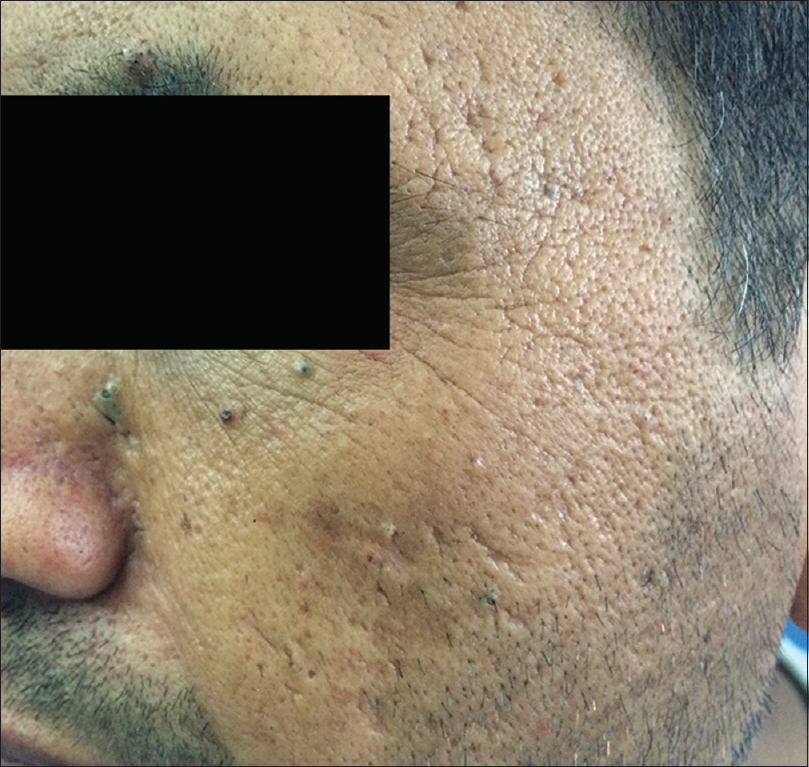 |
| Figure 5: Smokers comedones. Comedones in periorbital area and fine wrinkles in a 35-year-old man |
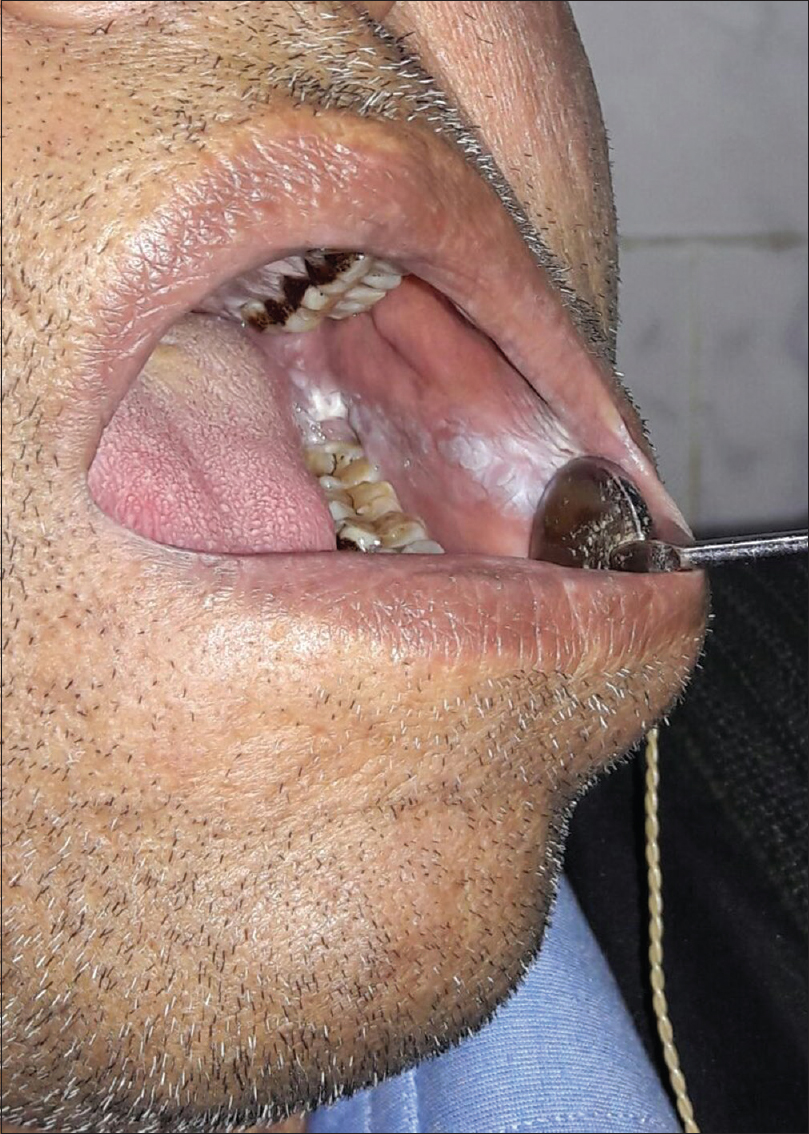 |
| Figure 6: Oral leukokeratosis in a smoker |
An association between cigarette smoke and psoriasis has been reported in several epidemiologic studies. In a Norwegian cross-sectional study, male smokers had a significantly increased risk of developing psoriasis.[40] A meta-analysis suggested that there is a significant association between smoking and psoriasis with a relative risk of 1.88 for smoking in patients with psoriasis versus patients without psoriasis.[41] In addition, there is a dose-dependent relationship between the development of psoriasis and the number of cigarettes smoked. A population-based twin study showed that childhood exposure to environmental tobacco smoke was significantly associated with psoriasis in the whole population, with an odds ratio of 1.28. Smokers with a history of >5 pack-years of cigarette had an increased risk of psoriasis with an odds ratio of 2.18. The same study showed that genetic factors could explain only 20% of the correlation between psoriasis and smoking, whereas non-shared environmental factors explained even less at 8 percent.[42] Several single nucleotide polymorphisms located in the CHRNA5/A3/B4 gene cluster have been linked to smoking behaviour and nicotine dependence, but these known single nucleotide polymorphisms were not found to be linked with psoriasis incidence or severity in the Chinese population.[43] The effect of smoking could be mediated by the reactive oxygen species and by the imbalance between oxidants and antioxidants indicated by low levels of vitamin C and glutathione, and high levels of superoxide dismutase and malonaldehyde.[3],[44]
Schäfer et al. reported a high prevalence of acne among smokers and described a correlation between the acne severity and the number of smoked cigarettes in post adolescent women, where predominantly comedonal acne was seen compared to the papulopustular form.[45] The authors reported that although the correlation between acne and smoking is still controversial, there is a hyperkeratinizing effect of cigarette smoke compounds, and in particular, of nicotine. Nicotine, an agonist of acetylcholine present in the cigarette, induces comedogenesis via the stimulation of acetylcholine-nicotinic receptor on epidermal keratinocytes.[46]
A meta-analysis done in 2010 shows that tobacco smoking is associated with cutaneous squamous cell carcinoma with an odds ratio of 1.52 while the association between smoking and basal cell carcinoma and other nonmelanoma skin cancers is controversial.[3],[25],[47],[48],[49],[50] In mice models, 4-(methylnitrosoamino)-1-(3-pyridyl)-1-butanone acted as tumor initiator causing skin cancer and lung adenomas.[19]
Interestingly, many case series and case-control studies report that smoking is associated with a lower prevalence of aphthous ulcers, Behcet's disease, herpes labialis, Kaposi's sarcoma (in acquired immune deficiency syndrome) and pemphigus vulgaris.[49] The various skin diseases aggravated by smoking are tabulated in [Table - 3].

Polycyclic aromatic hydrocarbons
Polycyclic aromatic hydrocarbons are among the most widespread and dangerous organic pollutants.[20] Polycyclic aromatic hydrocarbons are absorbed on the surface of suspended particulate matter in the air in urban areas.[51] They are converted into quinines, redox-cycling chemicals that produce reactive oxygen species.[52] Irrespective of the route of entry in the human body, they are found in almost all the internal organs, especially in the lungs and digestive tract. The main source of atmospheric polycyclic aromatic hydrocarbon benzo(a) pyrene is residual wood burning, the other sources being automobile exhaust, diesel fumes, metallurgical industry, production of plastics, pesticides, dyes, cigarette smoke and smoke resulting from the combustion of organic material.[21]
Polycyclic aromatic hydrocarbons are associated with extrinsic skin aging, pigmentation, cancers and acneiform eruption. Melanocyte proliferation and skin pigmentation have been observed in mice.[53] Scrotal cell carcinoma due to coal soot in British chimney sweeps was reported in 1775, and polycyclic aromatic hydrocarbon was the carcinogen responsible for it. Coal soot is more carcinogenic than wood soot as it contains a higher amount of polycyclic aromatic hydrocarbons. Among polycyclic aromatic hydrocarbons, benzo(a) pyrene has been shown to cause nonmelanoma cancers whereas 7,12-dimethylbenz(a) anthracene is capable of inducing lymphoma in hamsters. Ultraviolet A enhances the carcinogenic action of benzo(a) pyrene.[54] However, Sowada et al. have demonstrated that resident skin flora, predominantly micrococci, can degrade benzo(a) pyrene, thus forming an innate mechanism of defense against polycyclic aromatic hydrocarbons.[55],[56]
Epoxides and diols produced by activated polycyclic aromatic hydrocarbons bind to deoxyribonucleic acid, leading to carcinogenesis.[7],[54] Polycyclic aromatic hydrocarbons can lead to acneiform eruptions due to the presence of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin which is a polyhalogenated aromatic hydrocarbon.[7] It is formed in any burning, waste incineration, metal production and fossil-fuel and wood combustion. Sorg et al. described chloracne in Viktor Yushchenko's poisoning with 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin in 2005.[57] Chloracne is a systemic toxic disease caused by the exposure to halogenated aromatic hydrocarbons. It is characterized by comedones and cysts mainly on the face (outer sides of the eye and behind the ears) and neck. Other manifestations of chloracne include fatigue, liver dysfunction, neuropathy and arthritis.[58]
Ground-level ozone
Ozone is a ubiquitous pollutant in the urban environment. Its concentrations in urban environment can range from 0.2 to 1.2ppm.[59] Mexico City has the highest ozone levels in the world. It is a gaseous oxidant that exists in the stratosphere and troposphere.[70],[50] Normally, the concentrations of ozone at ground-level are low. Ozone, after interaction with sunlight (ultraviolet radiation), hydrocarbons, volatile organic compounds and nitrogen oxides, forms a major active component of the photochemical smog.[7],[50]
The effect of ozone is mediated by its ability to induce oxidative stress. It leads to the formation of peroxides, aldehydes and lipid ozonation products, as a result of unsaturated fatty acid oxidation and damages the barrier function of epidermis. Thiele et al. reported that ozone causes a reduction in the level of antioxidants such as tocopherol (vitamin E) and ascorbic acid (vitamin C) and increases malondialdehyde, a lipid peroxidation product in murine skin causing impairment of barrier function and inflammation.[22],[60] In human skin, exposure to ozone caused a 70% decrease in vitamin E concentration in stratum corneum, and 50% reduction in skin microflora.[61] Ozone causes disturbed activity of matrix metalloproteinases. Ozone-induced inflammation is mainly mediated through redox-sensitive transcription factor, nuclear factor kappa B. In an in vitro study, cells exposed to ozone demonstrated a dose-dependent increase in p65 subunit nuclear expression as a marker of nuclear factor kappa B activation, while pretreatment with vitamin C mixtures which acted as antioxidants abolished nuclear factor kappa B nuclear translocation. In addition, a significant activation of Nrf2 was observed in keratinocytes treated with the mixtures.[62] Nrf2 is a basic leucine zipper protein that regulates the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation.
Ozone along with ultraviolet A rays and cigarette smoke is a powerful oxidizing agent of squalene. Oxidation of squalene produces squalene by-products, mostly peroxidized forms, which lead to comedogenesis, thus aggravating inflammatory acne. Oxidation of epidermal lipids and disturbed activity of matrix metalloproteinases contribute to wrinkling and extrinsic skin aging.[63] Tropospheric ozone exposure has been associated with urticaria, eczema, contact dermatitis, rashes and infected skin disease.[64]
Particulate matter
Particulate matter in the air consists of complex and varying mixtures of different size and composition. Factories, power plants, refuse incinerators, automobile, construction activities, fires and natural windblown dust are some of the main sources of particulate matter.[65],[66]
Particulate matter penetrates skin either through hair follicles or transdermally, and exerts its detrimental effects through the generation of oxidative stress, which contributes to extrinsic skin aging, characterized particularly by pigment spots on the face and nasolabial folds, and less so by coarse wrinkles, solar elastosis and telangiectasia.[67],[68],[69] The most harmful components of ambient particulate matter are nanosize particles from traffic sources; these particles can serve as carriers for organic chemicals and metals that are capable of localizing in mitochondria and generating reactive oxygen species. It has been noted that an increase in soot (per 0.5 × 10−5/m) and particles from traffic (per 475 kg per year and square km) was associated with 20% more pigment spots on the forehead and cheeks. Polycyclic aromatic hydrocarbons are adsorbed on the surface of suspended particulate matter in air of urban areas.[68] Polycyclic aromatic hydrocarbons can activate xenobiotic metabolism which converts polycyclic aromatic hydrocarbons to quinones. Quinones are redox-cycling chemicals which produce reactive oxygen species responsible for particulate matter toxicity.[52]
Although many cohort studies report that there is no association between air pollutants and incidence and prevalence of atopic dermatitis, the severity of symptoms of atopic dermatitis may have a direct association with increased particulate matter.[70] Kim et al. also report that with indoor air quality improvement program, the level of particulate matter decreased and there was a significant decrease in the prevalence and severity of atopic dermatitis.[71] The exact mechanism is unclear, but it is proposed that particulate matter may induce inflammation in the skin in a similar fashion as that in the respiratory system.[70]
Volatile organic compounds
Emission of volatile organic compounds occurs from the use of organic solvents in paints, varnishes (aliphatic hydrocarbons, ethyl acetate, glycol ethers, methylene chloride and acetone) vehicle refinishing products in repairing car paint, environmental tobacco smoke, stored fuels, exhaust from cars (benzene) and from emissions from industrial facilities (tetrachloroethylene).[6],[72],[73] It is an important indoor source of air pollutants. A longitudinal study has shown that symptoms of atopic dermatitis increase in children shifted to a new building due to an increase in exposure to volatile organic compounds.[74] Volatile organic compounds along with sunlight and nitrogen oxides form photochemical oxidant products such as ozone at ground level which is the summer photochemical smog. Volatile organic compounds (ingestion of hexachlorobenzene) may induce precancerous skin lesions in rats.[75] Exposure to volatile organic compounds increases cytokines (interleukin-8 and interleukin-1B) in cultured keratinocytes which cause atopic dermatitis or eczema.
Oxides
Nitrogen oxides are emitted mainly from mobile and stationary combustion sources. They react with ozone-forming nitrogen dioxide. Among nitrogen oxides, nitrogen dioxide causes oxidative damage leading to the formation of free radicals that oxidize amino acids in tissue proteins and initiate lipid peroxidation of polyunsaturated fatty acids.[76]
Atmospheric sulfur dioxide is formed from fuel combustion from industrial processes, volcanic activity and forest fires. Carbon monoxide, a product of incomplete combustion from mobile sources, acts on cell metabolism which binds to heme and alters its function.[6] Flexural eczema was associated with traffic-related air pollutants, including nitrogen oxides and carbon monoxide in Taiwan in middle-school children. A study comparing atopic eczema in East and West Germany showed that the prevalence was higher in East Germany (sulfurous type pollution) and also exhibited a stronger association with nitrogen oxides and close proximity to heavy traffic.[77]
Heavy metals
Cadmium, lead and mercury are common air pollutants that pose health hazards. The main sources are volcanoes, waste incineration, cement, iron and steel production and leaded gasoline.[56]
Prevention Strategies
Control of air pollution is necessary to improve the health conditions. There are two steps in the prevention of dermatological diseases due to air pollution: the first is to reduce air pollution and the second is to use strategies to protect oneself from pollutants. In Korea, indoor air quality improvement program was conducted in nine kindergarten classes, following which mean particulate matter 10 levels decreased significantly from 182.7 to 73.4 μg/m [3]. Along with that, the prevalence, severity of atopic dermatitis in children and the number of hospital visits per month also decreased significantly, thus showing the benefit of improvement of air quality.[71] The Health Event project in Europe for the improvement of indoor air quality is based on three components, namely optimizing ventilation rates, filtration of outdoor air and indoor source control.[78] This project has shown an improvement in different cardiovascular and respiratory diseases. Such strategies should be studied for dermatological diseases too.
Control of air pollution
The natural sources of pollution are difficult to predict and prevent such as volcano eruptions or forest fires. However, human-made sources can be controlled. Some strategies include less use of personal vehicles, increase in the use of car pools and public modes of transport, supply of low-sulphur petrol, shifting of industries to areas away from the cities, development and usage of industrial machines and methods which are eco-friendly, avoiding burning of garbage in the open, avoidance of smoking and no use of wood and crop residues as fuel for the purpose of household cooking and heating. Various methods have been tried to reduce traffic induced pollution. For example, in New Delhi, biofriendly fuels such as compressed natural gas is used by all public transport vehicles, odd-even formula for private vehicles has been implemented and old diesel vehicles are gradually being phased out.
Personal protection
Strategies for personal protection include physical photoprotection; use of sunscreens; avoidance of areas with public smoking, and around industries; usage of topical antioxidants such as vitamin C and E in formulations along with sunscreen; and of indoor air purifiers and ventilators. People with high occupational risk, such as traffic police and sweepers, should use masks while at work.
Conclusions
Skin is the largest organ of human body, and any factor affecting skin health will impact the body as a whole. Major air pollutants having detrimental effects on the skin include solar ultraviolet radiation, polycyclic aromatic hydrocarbons, volatile organic compounds, nitrogen oxides, particulate matter, ozone and cigarette smoke. Sunlight, cigarette smoke and ambient particulate matter have a role to play in extrinsic skin aging. Smoking has also been associated with skin cancer, psoriasis, acne and skin malignancy. Exposure to ozone has been associated with urticaria, eczema, contact dermatitis and other nonspecific eruptions. Polyaromatic hydrocarbons cause skin cancer, extrinsic skin aging, pigmentation and acneiform eruptions. Oxides have been associated with increased prevalence, as well as exacerbations of atopic dermatitis in children.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide, Global Update 2005, Summary of Risk Assessment; 2006. Available from: http://www.who.int/phe/health_topics/outdoorair/outdoorair_aqg/en/. [Last accessed on 2016 Apr 04].
[Google Scholar]
|
| 2. |
Rizwan S, Nongkynrih B, Gupta SK. “Air pollution in Delhi: Its magnitude and effects on health”. Indian J Community Med 2013;38:4-8.
[Google Scholar]
|
| 3. |
Valacchi G, Sticozzi C, Pecorelli A, Cervellati F, Cervellati C, Maioli E. Cutaneous responses to environmental stressors. Ann N Y Acad Sci 2012;1271:75-81.
[Google Scholar]
|
| 4. |
Halliwell B, Cross CE. Oxygen-derived species: Their relation to human disease and environmental stress. Environ Health Perspect 1994;102 Suppl 10:5-12.
[Google Scholar]
|
| 5. |
Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, et al. Arsenic: Toxicity, oxidative stress and human disease. J Appl Toxicol 2011;31:95-107.
[Google Scholar]
|
| 6. |
Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut 2008;151:362-7.
[Google Scholar]
|
| 7. |
Baudouin C, Charveron M, Tarroux R, Gall Y. Environmental pollutants and skin cancer. Cell Biol Toxicol 2002;18:341-8.
[Google Scholar]
|
| 8. |
Kohen R, Gati I. Skin low molecular weight antioxidants and their role in aging and in oxidative stress. Toxicology 2000;148:149-57.
[Google Scholar]
|
| 9. |
National Ambient Air Quality Standards by Central Pollution Control Board 2009, New Delhi; 2009. Available from: http://www.envfor.nic.in/division/air-pollution. [Last accessed on 2016 Apr 04].
[Google Scholar]
|
| 10. |
Air Pollution in India, Real-time Air Quality Index. Available from: http://www.aqicn.org/map/india/. [Last accessed on 2016 Apr 04].
[Google Scholar]
|
| 11. |
Dessinioti C, Antoniou C, Katsambas A, Stratigos AJ. Basal cell carcinoma: What's new under the sun. Photochem Photobiol 2010;86:481-91.
[Google Scholar]
|
| 12. |
Yaar M, Gilchrest BA. Skin aging: Postulated mechanisms and consequent changes in structure and function. Clin Geriatr Med 2001;17:617-30, v.
[Google Scholar]
|
| 13. |
Gilchrest BA, Murphy GF, Soter NA. Effect of chronologic aging and ultraviolet irradiation on Langerhans cells in human epidermis. J Invest Dermatol 1982;79:85-8.
[Google Scholar]
|
| 14. |
Gilchrest BA, Stoff JS, Soter NA. Chronologic aging alters the response to ultraviolet-induced inflammation in human skin. J Invest Dermatol 1982;79:11-5.
[Google Scholar]
|
| 15. |
Flament F, Bazin R, Laquieze S, Rubert V, Simonpietri E, Piot B. Effect of the sun on visible clinical signs of aging in Caucasian skin. Clin Cosmet Investig Dermatol 2013;6:221-32.
[Google Scholar]
|
| 16. |
Adachi H, Murakami Y, Tanaka H, Nakata S. Increase of stratifin triggered by ultraviolet irradiation is possibly related to premature aging of human skin. Exp Dermatol 2014;23 Suppl 1:32-6.
[Google Scholar]
|
| 17. |
Aubin F. Mechanisms involved in ultraviolet light-induced immunosuppression. Eur J Dermatol 2003;13:515-23.
[Google Scholar]
|
| 18. |
Fabbrocini G, Triassi M, Mauriello MC, Torre G, Annunziata MC, De Vita V, et al. Epidemiology of skin cancer: Role of some environmental factors. Cancers (Basel) 2010;2:1980-9.
[Google Scholar]
|
| 19. |
Abarca JF, Casiccia CC. Skin cancer and ultraviolet-B radiation under the Antarctic ozone hole: Southern Chile, 1987-2000. Photodermatol Photoimmunol Photomed 2002;18:294-302.
[Google Scholar]
|
| 20. |
English JS, Dawe RS, Ferguson J. Environmental effects and skin disease. Br Med Bull 2003;68:129-42.
[Google Scholar]
|
| 21. |
Burke KE, Wei H. Synergistic damage by UVA radiation and pollutants. Toxicol Ind Health 2009;25:219-24.
[Google Scholar]
|
| 22. |
Thiele JJ, Podda M, Packer L. Tropospheric ozone: An emerging environmental stress to skin. Biol Chem 1997;378:1299-305.
[Google Scholar]
|
| 23. |
Sabziparvar AA, Shine KP, Forster PM. A model-derived global climatology of UV irradiation at the earth's surface. Photochem Photobiol 1999;69:193-202.
[Google Scholar]
|
| 24. |
Chow CK. Cigarette smoking and oxidative damage in the lung. Ann N Y Acad Sci 1993;686:289-98.
[Google Scholar]
|
| 25. |
Boyd AS, Shyr Y, King LE Jr. Basal cell carcinoma in young women: An evaluation of the association of tanning bed use and smoking. J Am Acad Dermatol 2002;46:706-9.
[Google Scholar]
|
| 26. |
Dietrich M, Block G, Norkus EP, Hudes M, Traber MG, Cross CE, et al. Smoking and exposure to environmental tobacco smoke decrease some plasma antioxidants and increase gamma-tocopherol in vivo after adjustment for dietary antioxidant intakes. Am J Clin Nutr 2003;77:160-6.
[Google Scholar]
|
| 27. |
Jorgensen LN, Kallehave F, Christensen E, Siana JE, Gottrup F. Less collagen production in smokers. Surgery 1998;123:450-5.
[Google Scholar]
|
| 28. |
Just M, Ribera M, Monsó E, Lorenzo JC, Ferrándiz C. Effect of smoking on skin elastic fibres: Morphometric and immunohistochemical analysis. Br J Dermatol 2007;156:85-91.
[Google Scholar]
|
| 29. |
Solly S. Clincial lectures on paralysis. Lancet 1856;68:641-3.
[Google Scholar]
|
| 30. |
Daniell HW. Smoker's wrinkles. A study in the epidemiology of “crow's feet”. Ann Intern Med 1971;75:873-80.
[Google Scholar]
|
| 31. |
Freiman A, Bird G, Metelitsa AI, Barankin B, Lauzon GJ. Cutaneous effects of smoking. J Cutan Med Surg 2004;8:415-23.
[Google Scholar]
|
| 32. |
O'Hare PM, Fleischer AB Jr., D'Agostino RB Jr., Feldman SR, Hinds MA, Rassette SA, et al. Tobacco smoking contributes little to facial wrinkling. J Eur Acad Dermatol Venereol 1999;12:133-9.
[Google Scholar]
|
| 33. |
Kadunce DP, Burr R, Gress R, Kanner R, Lyon JL, Zone JJ. Cigarette smoking: Risk factor for premature facial wrinkling. Ann Intern Med 1991;114:840-4.
[Google Scholar]
|
| 34. |
Urbanska M, Nowak G, Florek E. Cigarette smoking and its influence on skin aging. Przegl Lek 2012;69:1111-4.
[Google Scholar]
|
| 35. |
Thomsen SF, Sørensen LT. Smoking and skin disease. Skin Therapy Lett 2010;15:4-7.
[Google Scholar]
|
| 36. |
Chaichalotornkul S, Nararatwanchai T, Narkpinit S, Dararat P, Kikuchi K, Maruyama I, et al. Secondhand smoke exposure-induced nucleocytoplasmic shuttling of HMGB1 in a rat premature skin aging model. Biochem Biophys Res Commun 2015;456:92-7.
[Google Scholar]
|
| 37. |
Sorrentino JA, Krishnamurthy J, Tilley S, Alb JG Jr., Burd CE, Sharpless NE. p16INK4a reporter mice reveal age-promoting effects of environmental toxicants. J Clin Invest 2014;124:169-73.
[Google Scholar]
|
| 38. |
Yang GY, Zhang CL, Liu XC, Qian G, Deng DQ. Effects of cigarette smoke extracts on the growth and senescence of skin fibroblasts in vitro. Int J Biol Sci 2013;9:613-23.
[Google Scholar]
|
| 39. |
Kim JN, Kim HJ, Jeong SH, Kye YC, Son SW. Cigarette smoke-induced early growth response-1 regulates the expression of the cysteine-rich 61 in human skin dermal fibroblasts. Exp Dermatol 2011;20:992-7.
[Google Scholar]
|
| 40. |
Bø K, Thoresen M, Dalgard F. Smokers report more psoriasis, but not atopic dermatitis or hand eczema: Results from a Norwegian population survey among adults. Dermatology 2008;216:40-5.
[Google Scholar]
|
| 41. |
Richer V, Roubille C, Fleming P, Starnino T, McCourt C, McFarlane A, et al. Psoriasis and smoking: A systematic literature review and meta-analysis with qualitative analysis of effect of smoking on psoriasis severity. J Cutan Med Surg 2016;20:221-7.
[Google Scholar]
|
| 42. |
Lønnberg AS, Skov L, Skytthe A, Kyvik KO, Pedersen OB, Thomsen SF. Smoking and risk for psoriasis: A population-based twin study. Int J Dermatol 2016;55:e72-8.
[Google Scholar]
|
| 43. |
Zhu KJ, Liu Z, Liu H, Li SJ, Zhu CY, Li KS, et al. An association study on the CHRNA5/A3/B4 gene cluster, smoking and psoriasis vulgaris. Arch Dermatol Res 2014;306:939-44.
[Google Scholar]
|
| 44. |
Isik B, Ceylan A, Isik R. Oxidative stress in smokers and non-smokers. Inhal Toxicol 2007;19:767-9.
[Google Scholar]
|
| 45. |
Schäfer T, Nienhaus A, Vieluf D, Berger J, Ring J. Epidemiology of acne in the general population: The risk of smoking. Br J Dermatol 2001;145:100-4.
[Google Scholar]
|
| 46. |
Capitanio B, Sinagra JL, Bordignon V, Cordiali Fei P, Picardo M, Zouboulis CC. Underestimated clinical features of postadolescent acne. J Am Acad Dermatol 2010;63:782-8.
[Google Scholar]
|
| 47. |
El-Hakim IE, Uthman MA. Squamous cell carcinoma and keratoacanthoma of the lower lip associated with “Goza” and “Shisha” smoking. Int J Dermatol 1999;38:108-10.
[Google Scholar]
|
| 48. |
Leonardi-Bee J, Ellison T, Bath-Hextall F. Smoking and the risk of nonmelanoma skin cancer: Systematic review and meta-analysis. Arch Dermatol 2012;148:939-46.
[Google Scholar]
|
| 49. |
McBride P, Olsen CM, Green AC. Tobacco smoking and cutaneous squamous cell carcinoma: A 16-year longitudinal population-based study. Cancer Epidemiol Biomarkers Prev 2011;20:1778-83.
[Google Scholar]
|
| 50. |
Madronich S, Wagner M, Groth P. Influence of tropospheric ozone control on exposure to ultraviolet radiation at the surface. Environ Sci Technol 2011;45:6919-23.
[Google Scholar]
|
| 51. |
Menichini E. Urban air pollution by polycyclic aromatic hydrocarbons: Levels and sources of variability. Sci Total Environ 1992;116:109-35.
[Google Scholar]
|
| 52. |
Penning TM. Dihydrodiol dehydrogenase and its role in polycyclic aromatic hydrocarbon metabolism. Chem Biol Interact 1993;89:1-34.
[Google Scholar]
|
| 53. |
Krutmann J, Jux B, Luecke S, Fritsche E, Abel J, Essel C, Rannug A. Involvement of arylhydrocarbon receptor (AhR-) signaling in skin melanogenesis. J Invest Dermatol 2008;128:S220.
[Google Scholar]
|
| 54. |
Kelfkens G, de Gruijl FR, van der Leun JC. Tumorigenesis by short-wave ultraviolet A: Papillomas versus squamous cell carcinomas. Carcinogenesis 1991;12:1377-82.
[Google Scholar]
|
| 55. |
Sowada J, Schmalenberger A, Ebner I, Luch A, Tralau T. Degradation of benzo[a] pyrene by bacterial isolates from human skin. FEMS Microbiol Ecol 2014;88:129-39.
[Google Scholar]
|
| 56. |
Mancebo SE, Wang SQ. Recognizing the impact of ambient air pollution on skin health. J Eur Acad Dermatol Venereol 2015;29:2326-32.
[Google Scholar]
|
| 57. |
Sorg O, Zennegg M, Schmid P, Fedosyuk R, Valikhnovskyi R, Gaide O, et al. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) poisoning in Victor Yushchenko: Identification and measurement of TCDD metabolites. Lancet 2009;374:1179-85.
[Google Scholar]
|
| 58. |
Tindall JP. Chloracne and chloracnegens. J Am Acad Dermatol 1985;13:539-58.
[Google Scholar]
|
| 59. |
Mustafa MG. Biochemical basis of ozone toxicity. Free Radic Biol Med 1990;9:245-65.
[Google Scholar]
|
| 60. |
Thiele JJ, Traber MG, Tsang K, Cross CE, Packer L.In vivo exposure to ozone depletes Vitamins C and E and induces lipid peroxidation in epidermal layers of murine skin. Free Radic Biol Med 1997;23:385-91.
[Google Scholar]
|
| 61. |
He QC, Tavakkol A, Wietecha K, Begum-Gafur R, Ansari SA, Polefka T. Effects of environmentally realistic levels of ozone on stratum corneum function. Int J Cosmet Sci 2006;28:349-57.
[Google Scholar]
|
| 62. |
Valacchi G, Sticozzi C, Belmonte G, Cervellati F, Demaude J, Chen N, et al. Vitamin C compound mixtures prevent ozone-induced oxidative damage in human keratinocytes as initial assessment of pollution protection. PLoS One 2015;10:e0131097.
[Google Scholar]
|
| 63. |
Rittié L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev 2002;1:705-20.
[Google Scholar]
|
| 64. |
Xu F, Yan S, Wu M, Li F, Xu X, Song W, et al. Ambient ozone pollution as a risk factor for skin disorders. Br J Dermatol 2011;165:224-5.
[Google Scholar]
|
| 65. |
Pöschl U. Atmospheric aerosols: Composition, transformation, climate and health effects. Angew Chem Int Ed Engl 2005;44:7520-40.
[Google Scholar]
|
| 66. |
Dagouassat M, Lanone S, Boczkowski J. Interaction of matrix metalloproteinases with pulmonary pollutants. Eur Respir J 2012;39:1021-32.
[Google Scholar]
|
| 67. |
Lademann J, Schaefer H, Otberg N, Teichmann A, Blume-Peytavi U, Sterry W. Penetration of microparticles into human skin. Hautarzt 2004;55:1117-9.
[Google Scholar]
|
| 68. |
Vierkötter A, Schikowski T, Ranft U, Sugiri D, Matsui M, Krämer U, et al. Airborne particle exposure and extrinsic skin aging. J Invest Dermatol 2010;130:2719-26.
[Google Scholar]
|
| 69. |
Mills NL, Miller MR, Lucking AJ, Beveridge J, Flint L, Boere AJ, et al. Combustion-derived nanoparticulate induces the adverse vascular effects of diesel exhaust inhalation. Eur Heart J 2011;32:2660-71.
[Google Scholar]
|
| 70. |
Kim J, Kim EH, Oh I, Jung K, Han Y, Cheong HK, et al. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J Allergy Clin Immunol 2013;132:495-8.e1.
[Google Scholar]
|
| 71. |
Kim HO, Kim JH, Cho SI, Chung BY, Ahn IS, Lee CH, et al. Improvement of atopic dermatitis severity after reducing indoor air pollutants. Ann Dermatol 2013;25:292-7.
[Google Scholar]
|
| 72. |
Dales R, Liu L, Wheeler AJ, Gilbert NL. Quality of indoor residential air and health. CMAJ 2008;179:147-52.
[Google Scholar]
|
| 73. |
Okada Y, Nakagoshi A, Tsurukawa M, Matsumura C, Eiho J, Nakano T. Environmental risk assessment and concentration trend of atmospheric volatile organic compounds in Hyogo Prefecture, Japan. Environ Sci Pollut Res Int 2012;19:201-13.
[Google Scholar]
|
| 74. |
Kim EH, Kim S, Lee JH, Kim J, Han Y, Kim YM, et al. Indoor air pollution aggravates symptoms of atopic dermatitis in children. PLoS One 2015;10:e0119501.
[Google Scholar]
|
| 75. |
Michielsen CC, van Loveren H, Vos JG. The role of the immune system in hexachlorobenzene-induced toxicity. Environ Health Perspect 1999;107 Suppl 5:783-92.
[Google Scholar]
|
| 76. |
Eberlein-König B, Przybilla B, Kühnl P, Pechak J, Gebefügi I, Kleinschmidt J, et al. Influence of airborne nitrogen dioxide or formaldehyde on parameters of skin function and cellular activation in patients with atopic eczema and control subjects. J Allergy Clin Immunol 1998;101(1 Pt 1):141-3.
[Google Scholar]
|
| 77. |
Drakaki E, Dessinioti C, Antoniou CV. Air pollution and skin. Front Environ Sci 2014: 2;1-6.
[Google Scholar]
|
| 78. |
Asikainen A, Carrer P, Kephalopoulos S, Fernandes Ede O, Wargocki P, Hänninen O. Reducing burden of disease from residential indoor air exposures in Europe (HEALTHVENT project). Environ Health 2016;15 Suppl 1:35.
[Google Scholar]
|
Fulltext Views
53,336
PDF downloads
10,063





