Translate this page into:
Effects of isotretinoin on the thyroid gland and thyroid function tests in acne patients: A preliminary study
2 Department of Radiology, Faculty of Medicine, Sifa University, Izmir, Turkey
3 Department of Endocrinology, Faculty of Medicine, Sifa University, Izmir, Turkey
4 Department of Biochemistry, Faculty of Medicine, Sifa University, Izmir, Turkey
Correspondence Address:
Belkiz Uyar
35240, 172/2 Fevzipaşa Bulvarı Basmane, Izmir
Turkey
| How to cite this article: Uyar B, Solak A, Saklamaz A, Akyildiz M, Gen� B, G�kduman A. Effects of isotretinoin on the thyroid gland and thyroid function tests in acne patients: A preliminary study. Indian J Dermatol Venereol Leprol 2016;82:587-588 |
Abstract
Background: Isotretinoin is widely used in the treatment of acne. Aims: We investigated the effects of isotretinoin on thyroid function tests and thyroid volume in acne patients. Methods: In this prospective study, a total of 104 acne patients were included. Sixty-six patients were treated with isotretinoin for at least 4 months. Thirty eight patients were included in the control group. The levels of thyroid stimulating hormone, free triiodothyronine, free thyroxine, antithyroglobulin and antithyroid peroxidase antibodies were measured and a thyroid ultrasound was performed in all the subjects before treatment and 4 months after treatment. A “p” value of < 0.05 was considered significant. Results: In the isotretinoin-treated group, thyroid stimulating hormone levels increased significantly during isotretinoin treatment (P = 0.018). Free triiodothyronine, free thyroxine, anti-thyroid peroxidase levels and thyroid volume decreased significantly during treatment (P = 0.016, P= 0.012, P= 0.006, P = 0.020 respectively). Limitations: The major limitation of this study is the lack of follow-up data after the cessation of isotretinoin therapy in acne patients. Conclusion: Patients treated with isotretinoin should be monitored with thyroid function tests.Introduction
Isotretinoin (13-cis retinoic acid), a biologically active metabolite of vitamin A, has been used in the treatment of moderate or severe nodulocystic acne, disorders of sebaceous gland and keratinization and in the prevention of skin cancer.[1],[2],[3]
With the increasing use of isotretinoin, especially for the treatment of acne vulgaris and other disorders, the interest in the effect of this retinoid on other organs and the metabolic system has increased considerably.
The effect of vitamin A on the synthesis of thyroid hormone has been known for many years. In 1947, Simkins demonstrated successful treatment of patients with hyperthyroidism with a massive dose of vitamin A.[4] Our literature search revealed three reports about the effects of isotretinoin on thyroid function.[5],[6],[7] We evaluated the effects of isotretinoin on thyroid function tests and thyroid volume in acne patients with normal thyroid function. To our knowledge, this is the first clinical study investigating the effects of isotretinoin on both the thyroid function and thyroid volume
Methods
Patients
This prospective study included 104 patients who presented to our dermatology clinic with moderate or severe nodulocystic acne between April 2012 and December 2014. Among them, sixty-six patients were treated with isotretinoin for at least 4 months. A control group of 38 patients with moderate or severe nodulocystic acne, matched for age, gender and body mass index was recruited.
Patients with any of the following features were excluded from the study: history of smoking, abnormal blood pressure, body mass index >30 kg/m 2, current use of vitamin A supplements, previous therapy with oral retinoids or hormone therapy for any reason in the last 3 months, previously diagnosed thyroid or pituitary disease, pregnancy, coronary artery disease, diabetes mellitus, chronic renal failure and rheumatic disease. Patients with a recent history of psychiatric, mood or depressive disorders were also excluded from the study.
The baseline thyroid volumes of the subjects in both groups were within normal limits.
The study was approved by the local Medical Ethical Committee and was conducted according to the ethical principles of the Declaration of Helsinki. All the study patients gave written, informed consent to participate.
Isotretinoin therapy
Isotretinoin therapy was initiated at a dose of 0.5–0.8 mg/kg body weight. The drug was administered to acne patients twice daily with meals. Treatment was continued for at least 4 months. Patients in the control group did not receive treatment.
Biochemical parameters
In the study group, biochemical parameters and thyroid volume were screened prior to initiation (pre-treatment) and 4 months after the start of isotretinoin treatment (post-treatment). In the control group, biochemical parameters and thyroid volume were screened at the beginning of the study and repeated after 4 months. These parameters were: free triiodothyronine, free thyroxine, thyroid stimulating hormone, anti-thyroid peroxidase and antithyroglobulin.
Fasting blood samples were obtained by venepuncture of the large antecubital veins after a12-h fasting period. The samples were centrifuged immediately, the plasma separated and all samples were studied using the same kits. Free triiodothyronine (normal 2.3–4.2 pg/ml), free thyroxine (normal 0.98–1.63 ng/dl), thyroid stimulating hormone (normal 0.35–5.5 uU/ml), anti-thyroid peroxidase (normal 0–34 IU/ml) and antithyroglobulin (normal 5–115 IU/ml), were measured using electrochemiluminescent immunoassay methods.
Assessment of thyroid volume
Thyroid measurements were performed using a real-time ultrasound scanner with a 7.5 MHz, 50 mm linear transducer. Patients were examined in a supine position with the neck maximally extended.
Longitudinal and transverse scans of each thyroid lobe and isthmus were performed to obtain length, width and depth in centimeters. The thyroid volume was calculated by adding the volumes of each lobe and the isthmus. The lobar volume was calculated using the rotation ellipsoid model formula 14.15: VLobe (ml) = π/6 × width of the lobe (cm) × depth of lobe (cm) × length of the lobe (cm). The isthmus volume was calculated using the following formula: VIsthmus (ml) = π/6 × width of the isthmus (cm) × depth of isthmus (cm) × length of the isthmus (cm).
Statistical analyses
The normality of data was analyzed using the Kolmogorov–Smirnov test. All numerical variables with a normal distribution were expressed as the mean ± standard deviation while data that was not normally distributed was expressed as the median (interquartile range). For comparison between the pre- and post-treatment data of the treatment group and between the initial data and the data obtained after 4 months of the control group, the paired sample t-test was used for homogenous data. A Wilcoxon signed rank test was used for analysis of non-homogenous data. The statistical analyses were carried out using the Statistical Package for the Social Sciences version 20 (SPSS, Chicago, IL, USA).
Results
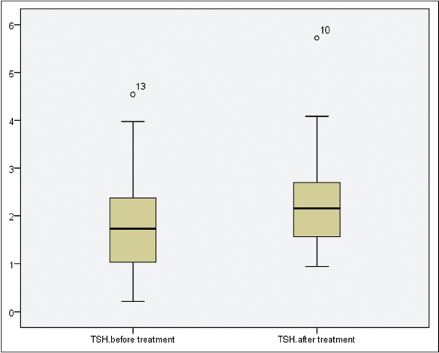
There were 14 (21.2%) males and 52 (78.8%) females in the isotretinoin-treated group and 8 (21.1%) males and 30 (78.9%) females in the control group not treated with isotretinoin. The mean age of the isotretinoin-treated group was 22.68 ± 4.51 years (age range of 18–36 years). The mean age of the control group was 23.82 ± 4.38 years (age range of 18–39 years). Comparisons of the thyroid function tests and thyroid volumes before and after isotretinoin treatment in the isotretinoin-treated group and the initial and later values in the control group are summarized in [Table - 1]. We found that levels of thyroid stimulating hormone (P = 0.018) increased significantly during isotretinoin treatment. Free triiodothyronine, free thyroxine and anti-thyroid peroxidase levels as well as thyroid volumes decreased significantly during treatment in acne patients (P = 0.016, P = 0.012, P = 0.006, P = 0.020 respectively). There was no significant difference in the levels of antithyroglobulin. Pre-treatment and post-treatment values of thyroid stimulating hormone [Figure - 1] and thyroid volume [Figure - 2] have been represented as box- plots.
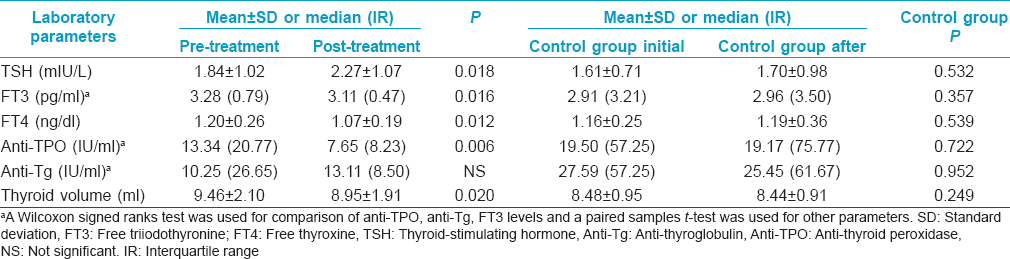
 |
| Figure 1: Pre- and post-treatment values of thyroid stimulating hormone levels are presented by box- plots graphics |
 |
| Figure 2: Pre- and post-treatment values of thyroid volume levels are presented by box- plots graphics |
In the control group, there was no statistically significant difference between the initial and later values of thyroid stimulating hormone, free triiodothyronine, free thyroxine, anti-thyroid peroxidase, antithyroglobulin levels and thyroid volume (P = 0.532, P = 0.357, P = 0.539, P = 0.722, P = 0.952, P = 0.249 respectively).
Discussion
It has long been known that high doses of vitamin A can interfere with the effects of thyroid hormone metabolism. Sadhu and Brody demonstrated a 10% decrease in oxygen consumption, a depressed metabolic rate and, interestingly, a 35% decrease of thyroid weight in euthyroid rats given high doses of vitamin A.[8]
The effect of vitamin A and retinoids on the hypothalamic-pituitary-thyroid axis has been well recognized but poorly understood.[9],[10]
Retinoids can affect cell growth, differentiation, function and metabolism via two groups of nuclear hormone receptors: retinoic acid receptors and retinoid X receptors. These receptors also mediate the activity of steroid and thyroid hormones.[11]
Recent studies have shown that hypothalamic-pituitary-thyroid function is primarily affected by retinoid X receptor-selective retinoids (called rexinoids).[12],[13]In vitro studies, animal models and human trials have demonstrated that rexinoids can suppress thyroid stimulating hormone.[12],[13],[14],[15],[16],[17] The effects of retinoids on thyroid function and thyrotrope function appear to be through a retinoid X receptor-mediated or rexinoid pathway.[9],[10],[12],[13],[16] Isotretinoin, a biologically active metabolite of vitamin A, is a weak retinoic acid receptor agonist. Janssen et al. suggested that although retinoid X receptor-selective retinoids suppress the thyroid stimulating hormone (TSH) level, retinoic acid receptor-selective retinoids such as isotretinoin may not suppress it.[18] The observation in our study that thyroid stimulating hormone levels increased in parallel with isotretinoin therapy supports the hypothesis that isotretinoin affects thyroid gland by different mechanisms than retinoid X receptor-selective retinoids.
A literature review revealed three clinical studies studying the effects of isotretinoin on thyroid function which had differing results.[5],[6],[7] The results of our study and previous trials examining the effects of isotretinoin treatment on thyroid gland are summarized in [Table - 2].

Marsden et al. studied seven patients with severe rosacea who received isotretinoin 1 mg/kg/day for 12 weeks and found a significant decrease in total thyroxine, free thyroxine index and total triiodothyronine. They found that mean total triiodothyronine declined from 2.2 nmol/L (pre-therapy) to 2.0 nmol/L at 12 weeks of therapy and continued to fall, reaching a nadir of 1.8 nmol/L 4 weeks after stopping therapy. They found no significant change in serum thyroid stimulating hormone (TSH) from the basal state.[5]
O'Leary et al. studied 24 women with acne vulgaris who received isotretinoin 1 mg/kg/day for 16 weeks. They studied serum free thyroxine, total triiodothyronine (before and 16 weeks after the therapy) and thyroid stimulating hormone (before and 8 weeks after the therapy). There was no significant change in serum free thyroxine. Like Marsden et al., they did not find a significant change in thyroid stimulating hormone with up to 8 weeks of therapy. In contrast, O'Leary et al. observed no significant change in total triiodothyronine.[6]
Karadag et al. initiated isotretinoin 0.5–0.75 mg/kg in 47 acne patients. They found that levels of free triiodothyronine, thyroid stimulating hormone and thyroid stimulating hormone receptor antibody decreased significantly at 3 months of isotretinoin therapy. Karadag et al. stated that there were no significant changes in the levels of thyroglobulin, antithyroglobulin or anti-thyroid peroxidase after commencing isotretinoin treatment.[7] In contrast to the above studies, our study revealed a statistically significant increase in thyroid-stimulating hormone (P = 0.018).
Several studies have indicated that isotretinoin and other retinoids affect cell cycle progression, differentiation, apoptosis and cell survival in a variety of cell types.[19],[20],[21],[22],[23],[24],[25] Interestingly, isotretinoin treatment of mice results in both decreased hippocampal neurogenesis and a reduction in the hippocampal volume.[23],[24] The reason for the increase in thyroid stimulating hormone observed in our study may be a reduction in thyroid volume as a result of the direct apoptotic effect of isotretinoin on the thyroid cells. Isotretinoin is known to increase iodine uptake in thyroid cancers, facilitating therapy.[26],[27],[28] This effect may also have induced volume reduction in the thyroid gland. As a result of the reduction in thyroid volume, free triiodothyronine and free thyroxine levels may have decreased and thyroid stimulating hormone level may have increased.
We enrolled patients with normal thyroid function. None of the patients experienced clinical symptoms of hypothyroidism such as increased sensitivity to cold, unexplained weight gain, puffy face, hoarseness, thinning hair, slowed heart rate or impaired memory after isotretinoin therapy for 4 months. Some of the symptoms were ignored because they might also occur as a result of isotretinoin treatment such as fatigue, dry skin, depression, muscle weakness, elevated blood cholesterol level, muscle aches, tenderness and stiffness and constipation. However, drug usage in patients who have thyroid dysfunction or are clinically hypothyroid may worsen metabolic pathology and induce irreversible hypothyroidism.
The major limitation of this study is the lack of follow-up data after the cessation of isotretinoin therapy. Another limitation is that we could not analyze men and women separately.
In conclusion, the increasing number of studies assessing the effects of isotretinoin on the thyroid gland will help clinicians to predict its side effects and to plan treatment accordingly. The next step in generating such data could possibly be expanding our study to include more patients and a longer follow-up.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Kraft J, Freiman A. Management of acne. CMAJ 2011;183:E430-5.
[Google Scholar]
|
| 2. |
Katz RA, Jorgensen H, Nigra TP. Elevation of serum triglyceride levels from oral isotretinoin in disorders of keratinization. Arch Dermatol 1980;116:1369-72.
[Google Scholar]
|
| 3. |
Peck GL, Gross EG, Butkus D, DiGiovanna JJ. Chemoprevention of basal cell carcinoma with isotretinoin. J Am Acad Dermatol 1982;6(4 Pt 2 Suppl):815-23.
[Google Scholar]
|
| 4. |
Simkins S. Use of massive doses of vitamin A in the treatment of hyperthyroidism: A preliminary report. J Clin Endocrinol Metab 1947;7:574-85.
[Google Scholar]
|
| 5. |
Marsden JR, Trinick TR, Laker MF, Shuster S. Effects of isotretinoin on serum lipids and lipoproteins, liver and thyroid function. Clin Chim Acta 1984;143:243-51.
[Google Scholar]
|
| 6. |
O'Leary TJ, Simo IE, Kanigsberg ND, Ooi TC. Lack of effect of isotretinoin on thyroid-function tests. Clin Chem 1986;32:913-4.
[Google Scholar]
|
| 7. |
Karadag AS, Ertugrul DT, Tutal E, Akin KO. Isotretinoin influences pituitary hormone levels in acne patients. Acta Derm Venereol 2011;91:31-4.
[Google Scholar]
|
| 8. |
Sadhu DP, Brody S. Excess vitamin A ingestion, thyroid size and energy metabolism. Am J Physiol 1947;149:400-3.
[Google Scholar]
|
| 9. |
Haugen BR. The effect of vitamin A, retinoids and retinoid receptors on the hypothalamic-pituitarythyroid axis. In:Beck-Peccoz P, editor. Syndromes of Hormone Resistance on the Hypothalamic-Pituitary-Thyroid Axis. Boston: Kluwer; 2004. p. 149-63.
[Google Scholar]
|
| 10. |
Morley JE, Melmed S, Reed A, Kasson BG, Levin SR, Pekary AE, et al. Effect of vitamin A on the hypothalamo-pituitary-thyroid axis. Am J Physiol 1980;238:E174-9.
[Google Scholar]
|
| 11. |
Evans RM. The steroid and thyroid hormone receptor superfamily. Science 1988;240:889-95.
[Google Scholar]
|
| 12. |
Sharma V, Hays WR, Wood WM, Pugazhenthi U, St Germain DL, Bianco AC, et al. Effects of rexinoids on thyrotrope function and the hypothalamic-pituitary-thyroid axis. Endocrinology 2006;147:1438-51.
[Google Scholar]
|
| 13. |
Sherman SI, Gopal J, Haugen BR, Chiu AC, Whaley K, Nowlakha P, et al. Central hypothyroidism associated with retinoid X receptor-selective ligands. N Engl J Med 1999;340:1075-9.
[Google Scholar]
|
| 14. |
Liu S, Ogilvie KM, Klausing K, Lawson MA, Jolley D, Li D, et al. Mechanism of selective retinoid X receptor agonist-induced hypothyroidism in the rat. Endocrinology 2002;143:2880-5.
[Google Scholar]
|
| 15. |
Dabon-Almirante CL, Damle S, Wadler S, Hupart K. Related case report: In vivo suppression of thyrotropin by 9-cis retinoic acid. Cancer J Sci Am 1999;5:171-3.
[Google Scholar]
|
| 16. |
Golden WM, Weber KB, Hernandez TL, Sherman SI, Woodmansee WW, Haugen BR. Single-dose rexinoid rapidly and specifically suppresses serum thyrotropin in normal subjects. J Clin Endocrinol Metab 2007;92:124-30.
[Google Scholar]
|
| 17. |
Smit JW, Stokkel MP, Pereira AM, Romijn JA, Visser TJ. Bexarotene-induced hypothyroidism: Bexarotene stimulates the peripheral metabolism of thyroid hormones. J Clin Endocrinol Metab 2007;92:2496-9.
[Google Scholar]
|
| 18. |
Janssen JS, Sharma V, Pugazhenthi U, Sladek C, Wood WM, Haugen BR. A rexinoid antagonist increases the hypothalamic-pituitary-thyroid set point in mice and thyrotrope cells. Mol Cell Endocrinol 2011;339:1-6.
[Google Scholar]
|
| 19. |
Pomponi F, Cariati R, Zancai P, De Paoli P, Rizzo S, Tedeschi RM, et al. Retinoids irreversibly inhibit in vitro growth of Epstein-Barr virus-immortalized B lymphocytes. Blood 1996;88:3147-59.
[Google Scholar]
|
| 20. |
Toma S, Isnardi L, Raffo P, Dastoli G, De Francisci E, Riccardi L, et al. Effects of all-trans-retinoic acid and 13-cis-retinoic acid on breast-cancer cell lines: Growth inhibition and apoptosis induction. Int J Cancer 1997;70:619-27.
[Google Scholar]
|
| 21. |
Cariati R, Zancai P, Quaia M, Cutrona G, Giannini F, Rizzo S, et al. Retinoic acid induces persistent, RARalpha-mediated anti-proliferative responses in Epstein-Barr virus-immortalized b lymphoblasts carrying an activated C-MYC oncogene but not in Burkitt's lymphoma cell lines. Int J Cancer 2000;86:375-84.
[Google Scholar]
|
| 22. |
Crandall J, Sakai Y, Zhang J, Koul O, Mineur Y, Crusio WE, et al. 13-cisretinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proc Natl Acad Sci U S A 1997;101:5111-6.
[Google Scholar]
|
| 23. |
Sakai Y, Crandall JE, Brodsky J, McCaffery P. 13-cis retinoic acid (accutane) suppresses hippocampal cell survival in mice. Ann N Y Acad Sci 2004;1021:436-40.
[Google Scholar]
|
| 24. |
Crandall J, Sakai Y, Zhang J, Koul O, Mineur Y, Crusio WE, et al. 13-cis-retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proc Natl Acad Sci U S A 2004;101:5111-6.
[Google Scholar]
|
| 25. |
Giannini F, Maestro R, Vukosavljevic T, Pomponi F, Boiocchi M. All-trans, 13-cis and 9-cis retinoic acids induce a fully reversible growth inhibition in HNSCC cell lines: Implications for in vivo retinoic acid use. Int J Cancer 1997;70:194-200.
[Google Scholar]
|
| 26. |
Grünwald F, Menzel C, Bender H, Palmedo H, Otte R, Fimmers R, et al. Redifferentiation therapy-induced radioiodine uptake in thyroid cancer. J Nucl Med 1998;39:1903-6.
[Google Scholar]
|
| 27. |
Simon D, Koehrle J, Reiners C, Boerner AR, Schmutzler C, Mainz K, et al. Redifferentiation therapy with retinoids: Therapeutic option for advanced follicular and papillary thyroid carcinoma. World J Surg 1998;22:569-74.
[Google Scholar]
|
| 28. |
Simon D, Körber C, Krausch M, Segering J, Groth P, Görges R, et al. Clinical impact of retinoids in redifferentiation therapy of advanced thyroid cancer: Final results of a pilot study. Eur J Nucl Med Mol Imaging 2002;29:775-82.
[Google Scholar]
|
Fulltext Views
18,735
PDF downloads
2,147





