Translate this page into:
Efficacy and safety of tofacitinib in patients with total and universal alopecia- A retrospective evaluation of 69 patients
Corresponding author: Dr. Dingquan Yang, Department of Dermatology, China-Japan Friendship Hospital, National Center for Integrated Traditional Chinese and Western Medicine, Beijing, China. ydqlx@163.com
-
Received: ,
Accepted: ,
How to cite this article: Lin W, Zhang F, Lv S, Wang Y, Yang D. Efficacy and safety of tofacitinib in patients with total and universal alopecia- A retrospective evaluation of 69 patients. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1755_2024
Abstract
Background
Alopecia areata (AA) is a chronic autoimmune dermatosis with significant challenges in the treatment of severe cases. Recently, tofacitinib has been a promising cure for moderate to severe AA. Yet, its long-term efficacy and safety in the treatment of alopecia totalis (AT) and alopecia universalis (AU) remain underexplored, and determinants of its effectiveness are poorly understood.
Aim
To evaluate the long-term efficacy and safety of tofacitinib in AT and AU patients and explore the factors that may influence treatment outcomes.
Methods
We retrospectively assessed the efficacy and safety of tofacitinib in 69 AT and AU patients between January 2020 and June 2024. We also explored risk factors affecting the efficacy of tofacitinib using binary logistic regression analysis.
Results
After a median treatment of 6 months with tofacitinib, 47.8% (33/69) of patients had a severity of alopecia tool (SALT) score ≤ 20, and 26.1% (18/69) had complete hair regrowth. Patients showed more pronounced SALT score reductions and significant efficacy at weeks 24 and 36, compared to week 12 (P < 0.01). Binary logistic regression analysis revealed that younger initial age of AA (odds ratio (OR) = 1.063, 95% confidence interval (CI) 1.011-1.117, P = 0.017) and shorter treatment duration (OR = 1.249, 95% CI 1.065-1.465, P = 0.006) were significant risk factors associated with poorer efficacy of tofacitinib. Acneiform rash and folliculitis were the primary adverse effects.
Limitation
The single-center, retrospective study may be limited by data deficiencies and a small sample size.
Conclusion
Tofacitinib demonstrated significant efficacy in treating patients with AT and AU within a minimum of 6 months. Severe adverse reactions were not observed. Notably, the age of AA onset and the duration of tofacitinib treatment may be significant factors influencing its therapeutic outcomes.
Keywords
Efficacy and safety
retrospective study
risk factors
tofacitinib
total and universal alopecia
Introduction
Alopecia areata (AA) is a CD8+/NKG2D+ T-cell-dependent autoimmune disease with an estimated prevalence of 0.22% in the United States. Approximately, 5% of these cases progress to alopecia universalis (AU) or alopecia totalis (AT).1,2 Both AU and AT are challenging to treat and have a poor prognosis. The Janus kinase (JAK) and signal transducers and activators of transcription (STAT) pathways play a crucial role in the immunopathogenesis of AA.3 Tofacitinib, a JAK 1/3 inhibitor, has recently shown promising results for severe AA and those unresponsive to conventional therapies.4,5 However, there are limited data on the efficacy and safety of longer-term clinical applications of tofacitinib. Our study aimed to evaluate the efficacy and safety of tofacitinib in Chinese patients with AT and AU.
Methods
We retrospectively analysed data from AT and AU patients treated with tofacitinib from January 2020 to June 2024. All patients received tofacitinib for at least three months and were evaluated every 2-4 months. The Severity of Alopecia Tool (SALT) score was used to assess hair loss pre-treatment and at various intervals during treatment. A SALT score ≤ 20 was considered a significant improvement6 and served as the primary endpoint. Secondary endpoints included the percentage change in the SALT score4 from baseline at weeks 12, 24, and 36. We also monitored complete remission (CR) rates and adverse events (AEs).
Data analysis and graphing were performed using IBM SPSS Statistics 27 and GraphPad Prism 8.0. Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range [IQR]). Comparisons of rates among more than two groups were conducted using the Chi-square test, while comparisons of continuous variables were performed using one-way analysis of variance (ANOVA). Finally, a binary logistic regression model was employed to analyse the factors influencing the efficacy of tofacitinib.
Results
Patient demographics, clinical presentation, and treatment responses to tofacitinib
Our study enrolled 69 patients treated with tofacitinib, of whom 8 had AT and 61 had AU. The study meticulously documented the baseline demographic characteristics, clinical features, and treatment responses to tofacitinib in these patients [Table 1]. The majority of patients were female (68.1%), with a mean age of 28.28 years and a body mass index (BMI) of 23.85 kg/m.2 The median SALT score before treatment was 100, and 75.4% were at the S5 severity level. The mean age of AA onset was 21.69 years, and the median duration of the current AA episode was 2 years. The median total disease duration was 4 years. The most commonly affected extra-scalp areas were eyebrows (72.5%) and eyelashes (68.1%), with a median baseline Clinician-Reported Outcome (ClinRO) score of 3. The most common comorbidities were allergic rhinitis (n = 10) and eczema (n = 10). Fourteen patients had previously received systemic corticosteroid therapy but discontinued it due to side effects and recurrences. The median duration of tofacitinib administration was 6 months, resulting in a median SALT score reduction to 30, a 66.67% decrease from baseline. Complete hair regrowth was achieved in 18 patients, while 13 showed no response. Among the patients with eyebrow and eyelash hair loss, the final median ClinRO scores were 1, with hair regrowth rates of 70% and 72.3%, respectively. AEs were reported in 25 (36.2%) patients, primarily as acneiform rashes (n = 13) and folliculitis (n = 9).
| Variables | Patients |
|---|---|
| Age, years, mean (SD) | 28.28 (9.37) |
| Sex, n (%) | |
| Male | 22 (31.9%) |
| Female | 47 (68.1%) |
| BMI, kg/m2, mean (SD) | 23.85 (4.82) |
| SALT grade, n (%) | |
| S3 | 7 (10.1%) |
| S4 | 10 (14.5%) |
| S5 | 52 (75.4%) |
| AT, n (%) | 8 (11.6%) |
| AU, n (%) | 61 (88.4%) |
| Baseline SALT score, median (IQR) | 100 (2.5) |
| Eyebrow hair loss, n (%) | 50 (72.5%) |
| Baseline ClinRO measure for eyebrow hair loss, median (IQR) | 3 (1) |
| Eyelash hair loss, n (%) | 47 (68.1%) |
| Baseline ClinRO measure for eyelash hair loss, median (IQR) | 3 (1) |
| Nail involvement, n (%) | 4 (5.8%) |
| Comorbidity, n (%) | |
| Allergic rhinitis | 10 (14.5%) |
| Eczema | 10 (14.5%) |
| Hypothyroidism | 2 (2.9%) |
| Atopic dermatitis | 2 (2.9%) |
| Age of onset of AA, years, mean (SD) | 21.69 (12.05) |
| Duration of AA, years, median (IQR) | 4 (8) |
| Duration of current episode of AA, years, median (IQR) | 2 (4) |
| Prior treatments, n (%) | |
| Systemic corticosteroids | 14 (20.3%) |
| Topical corticosteroids | 10 (14.5%) |
| Intralesional corticosteroids | 4 (5.8%) |
| Topical minoxidil | 11 (15.9%) |
| Concomitant treatments, n (%) | |
| Systemic corticosteroids | 26 (37.7%) |
| Topical corticosteroids | 57 (82.6%) |
| Intralesional corticosteroids | 28 (40.6%) |
| Topical minoxidil | 60 (87.0%) |
| Duration of treatment with tofacitinib, months, median (IQR) | 6 (6) |
| Most recent SALT score, median (IQR) | 30 (65) |
| SALT score changed after treatment, median (IQR) | 66.67 (70) |
| Most recent ClinRO measure for eyebrow hair loss, median (IQR) | 1 (2) |
| Most recent ClinRO measure for eyelash hair loss, median (IQR) | 1 (2) |
| Final treatment outcome, n (%) | |
| CR | 18 (26.1%) |
| Ineffectiveness | 13 (18.8%) |
| Eyebrow hair regrowth (complete or near-complete)* | 35 (70%) |
| Eyelash hair regrowth (complete or near-complete)* | 34 (72.3%) |
| AEs, n (%) | |
| Acneform eruptions | 13 (20.3%) |
| Epifolliculitis | 9 (13%) |
| Headache | 2 (2.9%) |
| Weight gain | 2 (2.9%) |
| Dyssomnia | 1 (1.4%) |
SD: Standard deviation, BMI: Body mass index, AT: Alopecia totalis, AU: Alopecia universalize, SALT: Severity of alopecia tool, IQR: Interquartile range, ClinRO: Clinician-reported outcome, CR: Complete hair regrowth achieved by treatment (S0), Ineffectiveness: SALT grade unchanged after treatment, *: ClinRO measure of 0 or 1, AEs: Adverse events.
Significant efficacy of tofacitinib and percent change of SALT score at weeks 12, 24, and 36
With a median treatment of 6 months, significant efficacy was achieved in 33 (47.8%) of the 69 patients by the end of the study. We analysed SALT scores at 12, 24, and 36 weeks to track the responses to tofacitinib. At these intervals, significant efficacy was achieved in 15.9%, 45.0%, and 68.2% of patients [Figure 1a]. The efficacies at weeks 24 and 36 were significantly higher than at week 12 (P < 0.001), and week 36 showed a trend toward even better results, though not statistically distinct from week 24 (P = 0.08). The median SALT score for each interval improved by 40%, 60%, and 90%, respectively, with significant differences between the groups [Figure 1b].
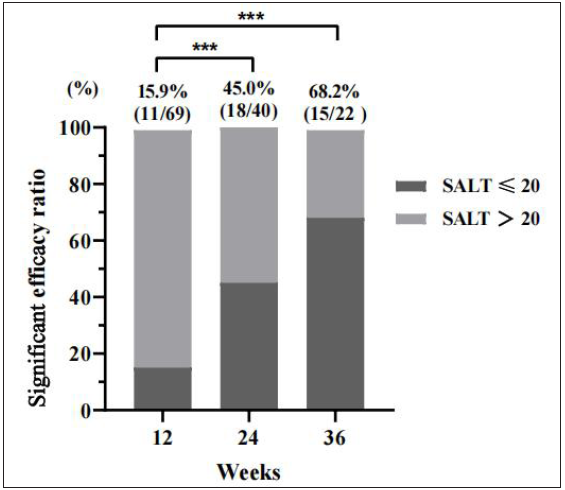
- Percentage of patients with significant efficacy (SALT score ≤ 20) at weeks 12, 24, and 36. Compared to weeks 12, ***P < 0.001.
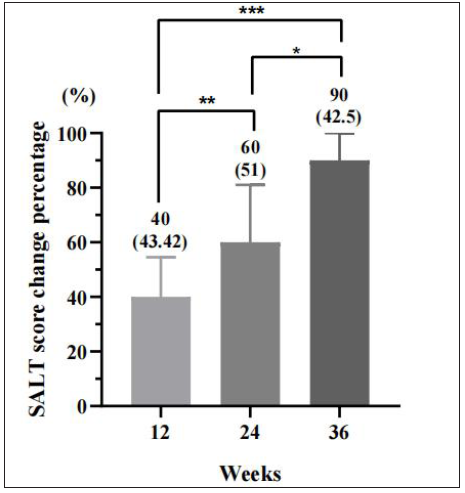
- Percentage of SALT score change at weeks 12, 24, and 36. Compared to weeks 12, *P < 0.05, **P < 0.01, ***P < 0.05.
Risk factors influencing the significant efficacy of tofacitinib
Univariate logistic regression analysis suggested that baseline SALT score, S4/S5 severity, age of AA onset, disease duration, and treatment duration could be included in the multivariate logistic regression analysis. With a significance level set at P < 0.1 among the above risk factors, only the age of AA onset (odds ratio (OR) = 1.063, 95% confidence interval (CI) 1.011-1.117, P = 0.017) and treatment duration (OR = 1.249, 95% CI 1.065-1.465, P = 0.006) were significant positive predictive factors for tofacitinib’s efficacy. Other variables, such as age, sex, baseline SALT score, and concomitant treatments did not significantly affect the outcome [Table 2].
| Variables | Univariate analyses | Multivariate analyses | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age | 1.048 (0.990-1.110) | 0.106 | - | - |
| Sex (Male/Famale) | 0.649 (0.229-1.838) | 0.432 | - | - |
| BMI | 1.085(0.949-1.241) | 0.232 | - | - |
| Baseline SALT score | 0.962 (0.921-1.005) | 0.086 | - | - |
| Baseline SALT grade (S3 as control) | ||||
| S4 | 0.111 (0.009-1.309) | 0.081 | - | - |
| S5 | 0.132 (0.015-1.177) | 0.070 | - | - |
| AT or AU | 0.906 (0.207-3.958) | 0.896 | - | - |
| Age at AA onset | 1.060 (1.011-1.112) | 0.017 | 1.063 (1.011-1.117) | 0.017 |
| Duration of AA | 0.925(0.847-1.010) | 0.082 | - | - |
| Duration of current episode of AA | 0.934(0.844-1.034) | 0.188 | - | - |
| Other hair loss involvement | 0.483(0.161-1.447) | 0.194 | - | - |
| Comorbidities | 1.600(0.490-5.228) | 0.437 | - | - |
| Prior treatments | ||||
| Systemic corticosteroids | 1.600 (0.490-5.228) | 0.437 | ||
| Intralesional corticosteroids | 3.500 (0.346-35.441) | 0.289 | ||
| Topical corticosteroids | 1.107(0.290-4.232) | 0.882 | ||
| Concomitant treatments | ||||
| Systemic corticosteroids | 0.898 (0.338-2.382) | 0.829 | ||
| Intralesional corticosteroids | 1.474 (0.561-3.871) | 0.431 | ||
| Topical corticosteroids | 0.391 (0.105-1.447) | 0.159 | ||
| Duration of treatment with tofacitinib | 1.227 (1.059-1.421) | 0.006 | 1.249 (1.065-1.465) | 0.006 |
Values in bold indicate ORs with a P < 0.1 in univariate analyses and P < 0.05 in multivariable analyses. OR: Odds ratio, CI: Confidence interval.
Discussion
Several studies have demonstrated the efficacy of tofacitinib as a monotherapy or combined therapy at a dose of 10 mg/day or higher for moderate to severe AA.5,7 A recent retrospective study8 showed that after 6 months of tofacitinib treatment, 45% of patients achieved a SALT score of S1, and the final CR rate was 33.75%. Our study also found that 45% of patients had a SALT score ≤ 20% after 6-month treatment, though ultimately, only 26.1% achieved CR. This discrepancy may be due to the inclusion of patchy AA or moderate AA in Huang et al.’s study8, while our study focused on patients with AT or AU SALT scores ≥ 50, which are considered refractory AA subtypes. Another study9, indicated a decrease from baseline SALT score of 84.5 to 48.6 after 9.8 months of continuous treatment with tofacitinib monotherapy in 9 AU patients. In our study, which included 61 (88.4%) AU patients, the baseline median SALT score of 100 was reduced by 66.67% to 30 after 24 weeks of tofacitinib treatment. While our results are encouraging, the potential synergistic effects of systemic or topical corticosteroids in the short or long term should not be ruled out. Furthermore, 72.5% and 68.1% of our study population had hair loss involving the eyebrow and eyelash areas, respectively. Encouragingly, the final results showed that over 70% of patients had achieved complete or near-complete hair regrowth in these areas. Our study indicated that tofacitinib for AT and AU could achieve significant clinical efficacy within 24 weeks, and that effects may be even better at 36 weeks or longer. During the treatment, 36.2% of patients reported AEs They primarily mentioned acneiform rashes and folliculitis, aligning with previous findings.5,8 These AEs did not lead to the discontinuation of tofacitinib or significantly affect its efficacy.
Our binary logistic risk assessment model revealed that variables such as AA episode duration, age, comorbidities, and concomitant treatments did not significantly impact the AT and AU patients’ response to tofacitinib. This finding differed from a previous study10, which suggested that a longer duration of AA episodes predicted a poorer response to JAK inhibitors. Our results indicated that the efficacy of tofacitinib was not significantly affected by the chronicity of AA, when considering multiple factors, while shorter treatment duration and younger age of AA onset were identified as predictors of less favourable outcomes. Eight of the 13 non-responders in our study stopped treatment within three months, which was the likely cause of the ineffective outcome. However, consistent with Mateos-Haro M et al. systematic review11 on tofacitinib’s efficacy and tolerability in paediatric AA, we believe that treatment with tofacitinib should not be limited by the age of onset. Timely and sustained treatment is crucial for refractory AA. Meanwhile, it is our duty to truthfully communicate the efficacy of tofacitinib, according to real-world studies, to patients, helping them set accurate psychological expectations and avoid blind optimism.
Limitations
This retrospective study relies on existing records, which may lack accuracy and completeness in patient data. Additionally, some key indicators affecting efficacy were under-monitored and under-recorded, such as tests for complete blood count (CBC), liver and kidney function, and immune/inflammatory markers.
Conclusion
This study demonstrates that tofacitinib represents a potentially effective alternative for AT and AU patients, with response occurring within six months in a high percentage of patients. It also exhibits a good safety profile, which is crucial for the long-term management of these chronic conditions. Furthermore, a younger age of AA onset and a shorter treatment duration may negatively impact the overall efficacy of tofacitinib. In light of these findings, clinicians and patients should be aware that early treatment initiation and longer treatment durations may enhance the efficacy.
Ethical approval
The study was approved by the Clinical Research Ethics Committee of China-Japan Friendship Hospital, number 2020-127-K80, dated 2020.12.23.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Trends in prevalence and incidence of alopecia areata, alopecia totalis, and alopecia universalis among adults and children in a US employer-Sponsored insured population. JAMA Dermatol. 2023;159:411-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Alopecia areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1-12.
- [CrossRef] [PubMed] [Google Scholar]
- An overview of JAK/STAT pathways and JAK inhibition in alopecia areata. Front Immunol. 2022;13:955035.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tofacitinib for the treatment of severe alopecia areata and variants: A study of 90 patients. J Am Acad Dermatol. 2017;76:22-8.
- [CrossRef] [PubMed] [Google Scholar]
- Oral tofacitinib and systemic corticosteroids, alone or in combination, in patients with moderate-to-Severe alopecia areata: A retrospective study. Front Med (Lausanne). 2022;9:891434.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Alopecia areata investigational assessment guidelines--Part II. National alopecia areata foundation. J Am Acad Dermatol. 2004;51:440-7.
- [Google Scholar]
- Comparison of the treatment outcome of oral tofacitinib with other conventional therapies in refractory alopecia totalis and universalis: A retrospective study. Acta Derm Venereol. 2019;99:41-6.
- [CrossRef] [PubMed] [Google Scholar]
- Drug survival and long-term outcome of tofacitinib in patients with alopecia areata: A retrospective study. Acta Derm Venereol. 2023;103:adv13475.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy of tofacitinib as an induction agent in severe alopecia areata compared with oral betamethasone weekly pulse. Clin Exp Dermatol. 2024;49:1236-8.
- [CrossRef] [PubMed] [Google Scholar]
- When to expect scalp hair regrowth during treatment of severe alopecia areata with baricitinib: Insights from trajectories analyses of patients enrolled in two phase III trials. Br J Dermatol. 2023;189:666-73.
- [CrossRef] [PubMed] [Google Scholar]
- Treatments for alopecia areata: A network meta-analysis. Cochrane Database Syst Rev. 2023;10:CD013719.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






