Translate this page into:
Efficacy of autologous platelet-rich plasma combined with fractional ablative carbon dioxide resurfacing laser in treatment of facial atrophic acne scars: A split-face randomized clinical trial
2 Skin and Stem Cell Research Center, Department of Dermatology, Tehran University of Medical Sciences, Tehran, Iran
Correspondence Address:
Shima Keyvan
Department of Dermatology, Al Zahra Hospital, Soffeh Blvd, 8174675731, Isfahan
Iran
| How to cite this article: Faghihi G, Keyvan S, Asilian A, Nouraei S, Behfar S, Nilforoushzadeh MA. Efficacy of autologous platelet-rich plasma combined with fractional ablative carbon dioxide resurfacing laser in treatment of facial atrophic acne scars: A split-face randomized clinical trial. Indian J Dermatol Venereol Leprol 2016;82:162-168 |
Abstract
Background: Autologous platelet-rich plasma has recently attracted significant attention throughout the medical field for its wound-healing ability. Aims: This study was conducted to investigate the potential of platelet-rich plasma combined with fractional laser therapy in the treatment of acne scarring. Methods: Sixteen patients (12 women and 4 men) who underwent split-face therapy were analyzed in this study. They received ablative fractional carbon dioxide laser combined with intradermal platelet-rich plasma treatment on one half of their face and ablative fractional carbon dioxide laser with intradermal normal saline on the other half. The injections were administered immediately after laser therapy. The treatment sessions were repeated after an interval of one month. The clinical response was assessed based on patient satisfaction and the objective evaluation of serial photographs by two blinded dermatologists at baseline, 1 month after the first treatment session and 4 months after the second. The adverse effects including erythema and edema were scored by participants on days 0, 2, 4, 6, 8, 15 and 30 after each session. Results: Overall clinical improvement of acne scars was higher on the platelet-rich plasma-fractional carbon dioxide laser treated side but the difference was not statistically significant either 1 month after the first treatment session (P = 0.15) or 4 months after the second (P = 0.23). In addition, adverse effects (erythema and edema) on the platelet-rich plasma-fractional carbon dioxide laser-treated side were more severe and of longer duration. Limitations: Small sample size, absence of all skin phototypes within the study group and lack of objective methods for the evaluation of response to treatment and adverse effects were the limitations. Conclusion: This study demonstrated that adding platelet-rich plasma to fractional carbon dioxide laser treatment did not produce any statistically significant synergistic effects and also resulted in more severe side effects and longer downtime.INTRODUCTION
Facial scarring caused by severe acne in adolescence and early adulthood is a common cosmetic problem accompanied by considerable psychological distress. The main cause of scarring is the formation of compromised collagen occurring during the natural wound-healing process. Different procedures have been used to influence dermal remodeling including surgical techniques (subcision, punch graft and punch excision), resurfacing techniques (dermabrasion, chemical peel, traditional ablative and non-ablative laser treatment) and tissue augmentation agents (autologous fat transfer and injection of dermal fillers). [1] In spite of these, treatment of acne scarring remains a challenge.
Facial resurfacing with fractional lasers is currently accepted to be one of the most effective treatments for facial scars. [2] Ablative fractional carbon dioxide lasers generate thermal energy targeting the separated columns of skin at specific depths called the microthermal treatment zones. [3],[4] This method intrinsically spares the tissue around the treated columns from damage allowing for prompt epidermal regeneration of the treated zones. [5] In addition, the enormous heat generated during ablative fractional carbon dioxide laser treatment can remove dermal tissue and bring about tissue shrinkage in the adjacent dermal collagen accompanied by collagen remodeling and skin tightening. [6]
Regardless of the profound effect of fractional carbon dioxide lasers in the treatment of acne scars, there are still some major concerns restricting the widespread use of this procedure. [7],[8],[9],[10],[11] These limitations include the risk of post-inflammatory hyperpigmentation, scarring and the longer post-procedure down time required that can disrupt patients′ daily lives.
Autologous platelet-rich plasma contains the plasma portion of autologously sourced blood with an iatrogenically high platelet concentration. [12] Recently, platelet-rich plasma has become popular in many areas of medicine including plastic surgery and dermatology for scar remodelling. [13],[14],[15],[16],[17] Platelets release growth factors including platelet-derived growth factor and transforming growth factor-β, facilitating and accelerating bone and soft tissue regeneration. [18],[19],[20],[21] We hypothesized that given its regenerative properties, platelet-rich plasma may augment the response of acne scars to fractional carbon dioxide laser treatment and minimize the post -procedure side effects. This study aimed to investigate the potential of the combination therapy with autologous platelet-rich plasma and fractional carbon dioxide laser in enhancing the treatment response of facial acne scars and reducing the risk of adverse events.
METHODS
Patients
A total of 16 patients (12 women, 4 men), aged 22-52 years (mean age: 36.8), of Fitzpatrick skin types II-IV with moderate to severe facial atrophic acne scars (predominantly rolling and boxcar types with fewer than 20% of the icepick type) were enrolled in this study. We used the grading system suggested by Goodman and Baron for qualitative grading of the participants′ acne scars. [22] Those with a history of keloid formation, herpes simplex infection, any active inflammation, diabetes mellitus, collagen vascular disease, oral isotretinoin use within the previous 6 months, pregnancy, lactation and ablative or non-ablative laser skin resurfacing in the previous 12 months were excluded. This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of Isfahan University of Medical Sciences. Written informed consent was obtained from all the participants. The study was registered at the Iranian Registry of Clinical Trials; www.irct.ir, registration number: IRCT2014080218648N1.
Platelet-rich plasma preparation
A two-stage centrifuging process was employed in the preparation of platelet-rich plasma. Whole blood samples (20 ml) were drawn from the participant′s medial cubital vein in a syringe and transferred to a tube prefilled with 2.4 ml anticoagulant (citrate phosphate dextrose) solution. The mixture was then centrifuged at 2000 g for 3 min. After the first spin, the lower red blood cell portion was discarded and the supernatant that contained platelet-poor plasma and buffy coat was centrifuged at 5000 g for 5 min. The resulting pellet of platelets (lower portion) was mixed with 4 ml of supernatant. This process produced approximately 4 ml of platelet-rich plasma. Three percent calcium chloride was added as platelet activator.
Treatment procedure
About 60 min before the starting the treatment, the targeted region was treated with topical anesthetic cream (mixture of lidocaine 2.5% and prilocaine 2.5%, Xyla P [Tehran Chemical Pharmaceutical Co., Tehran, Iran]) and icepacks to alleviate the pain followed by gentle cleansing and applicaton of 70% isopropyl alcohol as disinfectant.
Subsequently, the dermatologist (ShK) treated both the cheeks of each participant using the ablative carbon dioxide fractional laser (Q-ray, Diosis Inc., Seoul, Korea). In each treatment session, these parameters were used: laser power, 25; dot cycle (duration), 3; energy, 30mj, pixel pitch, 1 and ablation depth, 600 μm.
After the ablative carbon dioxide laser resurfacing treatment, each patient was administered autologous platelet-rich plasma on one half of the face (selected randomly) and normal saline was administered on the other half. Injection sites were located within 2 cm intervals to receive 0.2 ml platelet-rich plasma or normal saline. Afterwards, the participants were required to compress their faces with gauze for 15 min before leaving. Ice packs or analgesic medications were not used after treatment. All patients were asked to avoid direct sun exposure, heat and friction on the treated areas. They were instructed to apply a topical antibiotic (mupirocin) cream twice daily for 5 days after the treatment session and a broad spectrum sunscreen with a sun protection factor (SPF) of at least 30 every morning . One month after the initial treatment session, all participants received the second treatment session with the same protocol .
Patient assessment
Participants were required to undergo serial photography of the lesions at baseline, 1 month after the first treatment session and 4 months after the second, using a digital camera (Visio Face ® 1000, >12 megapixels, CK Electronic Inc., Germany). Camera setting, lighting and positioning were kept identical for all serial photographs. A quartile grading scale: poor, <25% improvement; fair, 25-50% improvement; good, 51-75% improvement and excellent, >75% improvement was used by two blinded dermatologists (GF and ShB who did not perform the procedures) to evaluate the overall clinical improvement.
Each participant was instructed to evaluate his/her overall satisfaction with the treatment 1 month after the first treatment session and 4 months after the last, using a quartile grading system which defines 0 as unsatisfied, 1 as slightly satisfied, 2 as satisfied, or 3 as very satisfied. The visual analog scale (scoring from 0 to 10) was used to record adverse events (erythema and edema) as perceived by participants on days 0, 2, 4, 6, 8, 15 and 30 after each treatment session.Finally, the occurrence of other possible side adverse events including secondary infection, acneiform eruption, dyschromia and new scar formation was assessed by a blinded dermatologist.
Statistical analysis
SPSS version 20 for Microsoft Windows was used for statistical analysis (SPSS Inc., Chicago, IL, USA).The Wilcoxon rank test was used to compare the results of the two methods for the degree of clinical improvement of acne scars and patient satisfaction. The paired t-test was utilized for group comparison of numerical variables (erythema and edema scores).P < 0.05 was considered statistically significant.
RESULTS
A total of 16 participants completed the study. Patient characteristics are shown in [Table - 1]. [Table - 2] shows the acne scar grades of the two facial halves of the patients.

Dermatologist′s evaluation
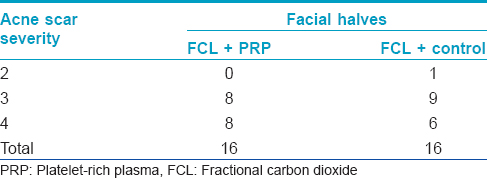
A quartile grading scale was used by two blinded dermatologists to assess the three serial photographs. A fair or good improvement was noted in 11 (68.8%) patients on the fractional carbon dioxide laser - platelet-rich plasma side and in 8 (50%) patients on the fractional carbon dioxide laser-normal saline side after the first treatment session (P = 0.15) [Figure - 1]. Four months after the second session, there was a fair or good response in 14 (87.5%) patients on the fractional carbon dioxide laser-platelet-rich plasma side and in 11 (68.8%) patients on the fractional carbon dioxide laser-normal saline side (P = 0.23) with none having an excellent outcome [Figure - 2]. [Figure - 3] shows the serial figures of one patient.
 |
| Figure 1: Dermatologists-assessed percentage of clinical improvement 1 month after the first treatment session |
 |
| Figure 2: Dermatologists-assessed percentage of clinical improvement 4 months after the second treatment session |
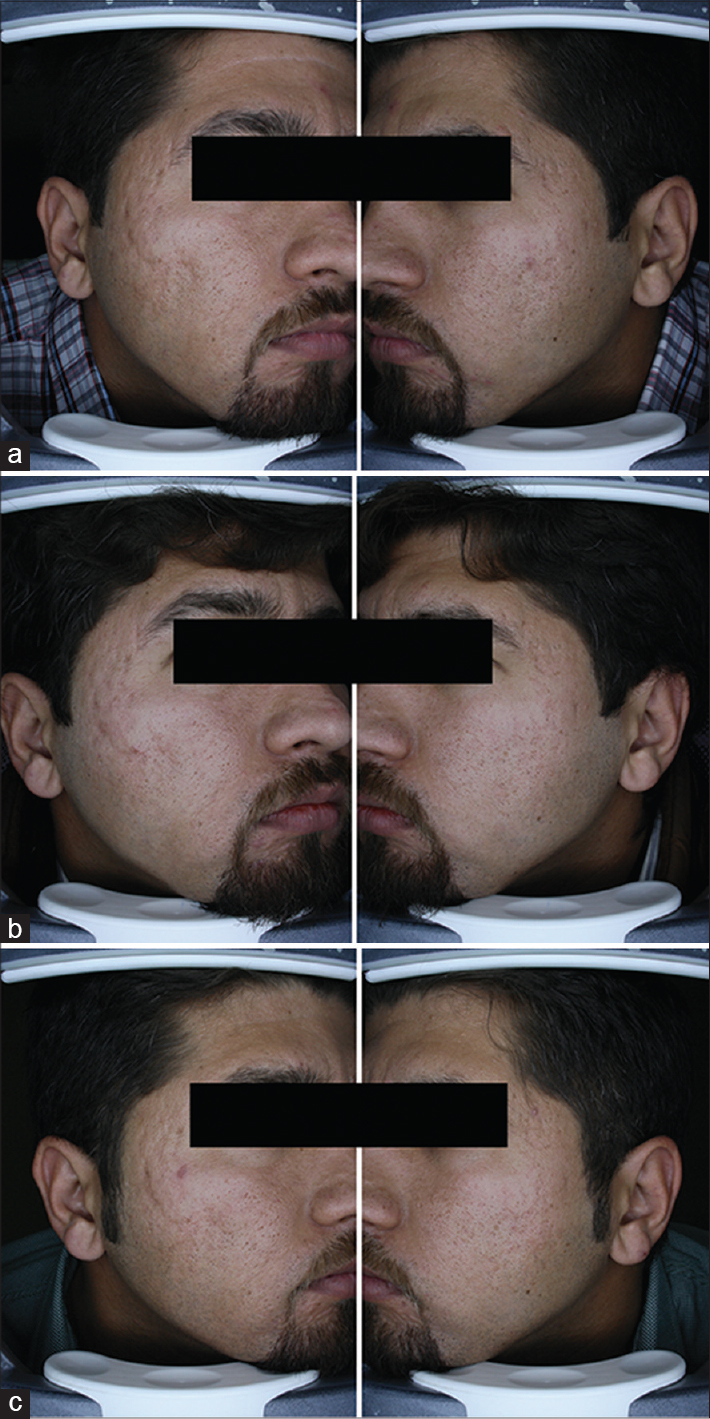 |
| Figure 3: Left side of picture: Platelet-rich plasma - fractional carbon dioxide side, right side of picture: Fractional carbon dioxide-alone side (a) baseline, severe acne scar (b) 1 month after first treatment session, left side was scored poor and right side fair and (c) 4 months after second treatment session. Left side improved to good score but right side was again scored fair |
Patient satisfaction
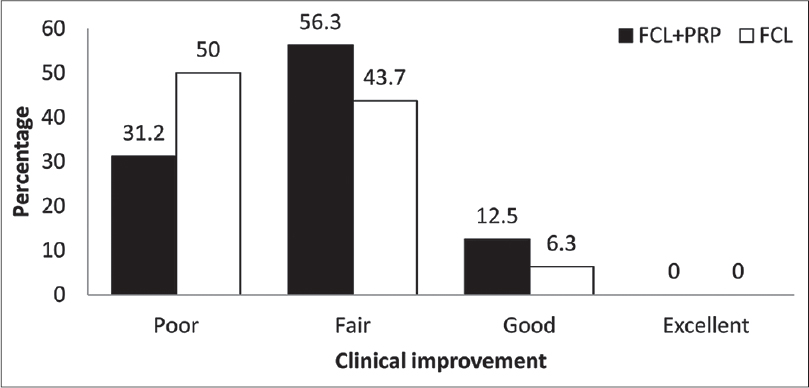
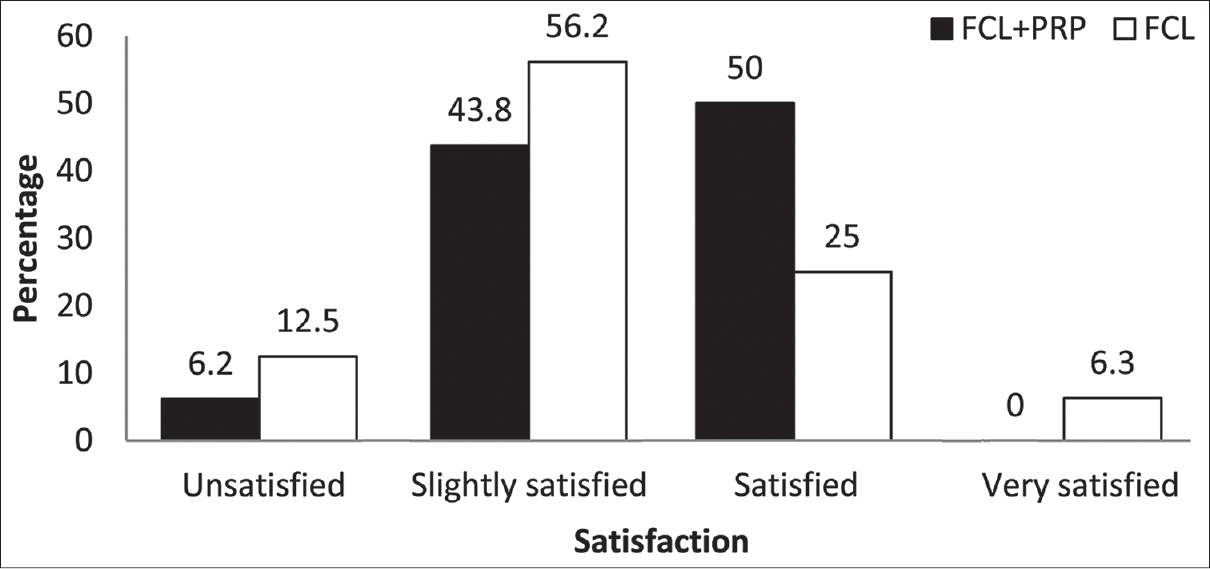
Eight (50%) patients stated that they were satisfied or very satisfied on the fractional carbon dioxide laser-platelet-rich plasma side, while five (31.2%) patients stated this about the fractional carbon dioxide laser-normal saline side 1 month after the first treatment session (P = 0.18) [Figure - 4]. In addition, 4 months after the second treatment session, nine patients (56.2%) reported being satisfied or very satisfied on the fractional carbon dioxide laser-platelet-rich plasma side while seven patients (43.8%) described the same result on the fractional carbon dioxide laser-control side (P = 0.12) [Figure - 5].
 |
| Figure 4: Percentage of patients' satisfaction 1 month after first treatment session |
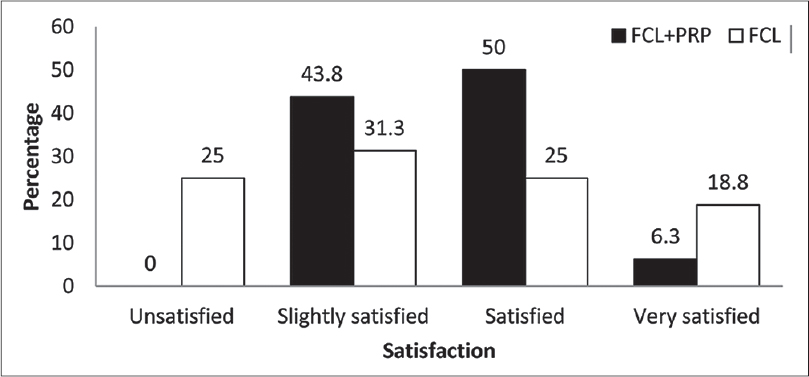 |
| Figure 5: Percentage of patients' satisfaction 4 months after second treatment session |
The fractional carbon dioxide laser-platelet-rich plasma side was found to have a better response than the fractional carbon dioxide laser-control side and participants were more satisfied with the fractional carbon dioxide laser-platelet-rich plasma side than the fractional carbon dioxide laser-control side; however, the difference was not found to be statistically significant.
Side effects
Participants recorded the severity of erythema and edema on a 0-10 visual analogue score on days 0, 2, 4, 6, 8, 10, 15, 30 after each treatment session. Paired t-tests revealed more severe erythema on the fractional carbon dioxide laser-platelet-rich plasma side than the fractional carbon dioxide laser-control side with significant statistical difference on days 0, 2 and 4 (P = 0.003, P = 0.007, P = 0.03, respectively), as shown in [Figure - 6] (these results are of mean values from first and second treatments). Paired t-tests also revealed more severe edema on the fractional carbon dioxide laser-platelet-rich plasma side compared with the fractional carbon dioxide laser-control side with a statistically significant difference on days 0, 2 and 8 (P = 0.003, P = 0.004, P = 0.004 respectively), as shown in [Figure - 7] (these results are of mean values from first and second treatments). The calculated mean duration of edema was about 6.3 ± 2.6 days on fractional carbon dioxide laser-platelet-rich plasma side and 5.1 ± 1.5 on fractional carbon dioxide laser-normal saline side (P = 0.12). In addition, erythema lasted an average of 4.9 ± 3.1 days on the fractional carbon dioxide laser-platelet-rich plasma side and 4.1 ± 2.9 on fractional carbon dioxide laser-normal saline side (P = 0.06) indicating longer duration of edema and erythema on the fractional carbon dioxide laser-platelet-rich plasma side even when there is no significant statistical difference in severity. There were no reports of other adverse events including secondary infection, acneiform eruption, dyschromia and new scar formation.
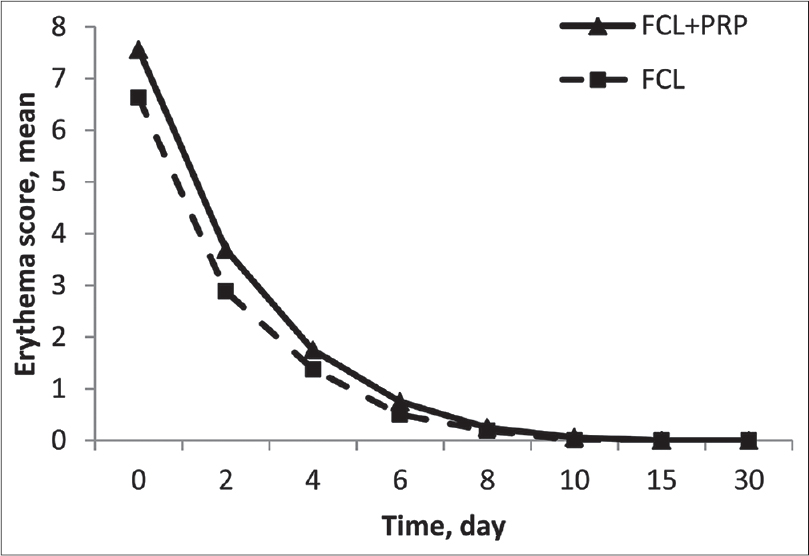 |
| Figure 6: Patients-assessed mean score of erythema in two groups (these results are of mean values from first and second treatment sessions) |
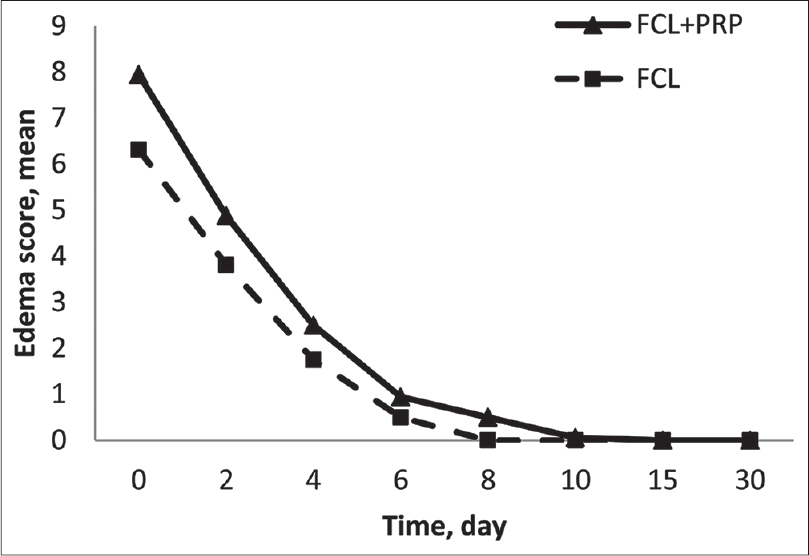 |
| Figure 7: Patients-assessed mean score of edema in two groups (these results are of mean values from first and second treatment sessions) |
DISCUSSION
Our results show that the combination of platelet-rich plasma and ablative fractional carbon dioxide lasers improved facial acne scars and resulted in greater patient satisfaction but not to a statistically significant level. Our study also showed that the adverse effects (erythema and edema) lasted longer and were more severe on the platelet-rich plasma-fractional carbon dioxide laser side when compared to the fractional carbon dioxide laser-normal saline side.
Recently, a few studies have looked into the effectiveness of platelet-rich plasma combined with ablative fractional carbon dioxide laser in the treatment of atrophic acne scars with most of them reporting that platelet-rich plasma caused greater clinical improvement and also reduced the laser side effects, erythema and edema. Lee et al. conducted a split-face trial on 14 Korean patients with facial atrophic acne scars who were treated with platelet-rich plasma following ablative fractional carbon dioxide laser. Patients received ablative fractional carbon dioxide laser on both sides of the face and subsequently, they randomly received intradermal platelet-rich plasma on one half of the face and intradermal normal saline on the other half. Overall clinical improvement was significantly enhanced on the intradermal platelet-rich plasma-side than the other side. In contrast to our study, they reported that erythema and edema on the intradermal platelet-rich plasma-side improved faster. In their study, these two side effects were evaluated by blinded dermatologists and also by an objective method using a chromometer to evaluate the intensity of erythema. [13]
The correlation between shorter down time and platelet-rich plasma following fractional carbon dioxide laser was demonstrated in another study as well. Gawdat et al. used topical and intradermal platelet-rich plasma after ablative fractional carbon dioxide laser for acne scar treatment in 30 patients. One group underwent treatment with fractional carbon dioxide laser-intradermal platelet-rich plasma on one side and fractional carbon dioxide laser-intradermal normal saline on the other side. The other group underwent fractional carbon dioxide laser with intradermal platelet-rich plasma on one side and fractional carbon dioxide laser with topical platelet-rich plasma on the other side. In addition to reporting synergistic effects on clinical response and patient satisfaction after adding platelet-rich plasma to fractional carbon dioxide laser, they also showed that side effects were fewer on the platelet-rich plasma sides (both intradermal and topical) than the other sides. [15] Other studies also reported less erythema after adding platelet-rich plasma to laser therapy. [23],[24]
There are several mechanisms that could explain the synergistic effects of platelet-rich plasma on overall clinical improvement. Activated platelets possess α-granules containing secretory proteins (e.g., platelet-derived growth factor and transforming growth factor). These proteins are bound to the receptors of target cells (e.g., mesenchymal stem cells, osteoblasts, fibroblasts, endothelial cells and epidermal cells) and direct cellular proliferation, matrix formation, osteoid production, collagen synthesis, etc. [25],[26] Platelet-derived growth factor, transforming growth factor-β and IGF also facilitate chemotaxis and mitogenesis of stem cells and osteoblasts, angiogenesis for capillary ingrowth, bone matrix formation and collagen synthesis. [27],[28],[29]
Another mechanism is the production of hyaluronic acid which can absorb water resulting in volume and skin turgor, stimulation of cell proliferation, extracellular matrix production and modulation of the diameter of collagen fibers. [24]
Our results show that although platelet-rich plasma can have a role in the treatment of atrophic acne scars, it has more local side effects. This worsening of erythema and edema could be attributed to the accumulating evidence that demonstrates that platelets contribute to the initiation and propagation of the inflammatory process. [30]
Study limitations
The limitations of our study include small sample size, absence of of all skin phototypes and the lack of objective methods for clinical evaluation of acne scar improvement and side effects. We feel that further studies are required to investigate the mechanism, benefits and safety of autologous blood-derived platelet-rich plasma.
CONCLUSION
Adding platelet-rich plasma to ablative fractional carbon dioxide laser resulted in non-significant synergistic effects with more severe side effects and longer downtime.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Acknowledgements
This study was supported by a grant from the Isfahan University of Medical Sciences (Grant no. 393020). The authors would like to acknowledge Dr. Ziba Farajzadegan and Dr. Bahareh Abtahi-Naeini who helped us edit this manuscript, Dr. Elahe Haftbaradaran for her assistance in performing procedures, Prof. Pourazar, head of Zistpartak laboratory, for platelet-rich plasma preparation and Dr. Homa Fatehi for help in patient selection.
Financial support and sponsorship
Isfahan University of Medical sciences (Grant no. 393020).
Conflicts of interest
There are no conflicts of interest.
| 1. |
Fabbrocini G, Annunziata MC, D′Arco V, De Vita V, Lodi G, Mauriello MC, et al. Acne scars: Pathogenesis, classification and treatment. Dermatol Res Pract 2010;2010:893080.
[Google Scholar]
|
| 2. |
Majid I, Imran S. Fractional CO2 Laser resurfacing as monotherapy in the treatment of atrophic facial acne scars. J Cutan Aesthet Surg 2014;7:87-92.
[Google Scholar]
|
| 3. |
Manstein D, Herron GS, Sink RK, Tanner H, Anderson RR. Fractional photothermolysis: A new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med 2004;34:426-38.
[Google Scholar]
|
| 4. |
Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: Nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol 2008;58:719-37.
[Google Scholar]
|
| 5. |
Chapas AM, Brightman L, Sukal S, Hale E, Daniel D, Bernstein LJ, et al. Successful treatment of acneiform scarring with CO2 ablative fractional resurfacing. Lasers Surg Med 2008;40:381-6.
[Google Scholar]
|
| 6. |
Hantash BM, Bedi VP, Chan KF, Zachary CB. Ex vivo histological characterization of a novel ablative fractional resurfacing device. Lasers Surg Med 2007;39:87-95.
[Google Scholar]
|
| 7. |
Cho SB, Lee SJ, Cho S, Oh SH, Chung WS, Kang JM, et al. Non-ablative 1550-nm erbium-glass and ablative 10 600-nm carbon dioxide fractional lasers for acne scars: A randomized split-face study with blinded response evaluation. J Eur Acad Dermatol Venereol 2010;24:921-5.
[Google Scholar]
|
| 8. |
Hedelund L, Haak CS, Togsverd-Bo K, Bogh MK, Bjerring P, Haedersdal M. Fractional CO2 laser resurfacing for atrophic acne scars: A randomized controlled trial with blinded response evaluation. Lasers Surg Med 2012;44:447-52.
[Google Scholar]
|
| 9. |
Manuskiatti W, Iamphonrat T, Wanitphakdeedecha R, Eimpunth S. Comparison of fractional erbium-doped yttrium aluminum garnet and carbon dioxide lasers in resurfacing of atrophic acne scars in Asians. Dermatol Surg 2013;39 (1 Pt 1):111-20.
[Google Scholar]
|
| 10. |
Zhang Z, Fei Y, Chen X, Lu W, Chen J. Comparison of a fractional microplasma radio frequency technology and carbon dioxide fractional laser for the treatment of atrophic acne scars: A randomized split-face clinical study. Dermatol Surg 2013;39:559-66.
[Google Scholar]
|
| 11. |
Fife DJ, Fitzpatrick RE, Zachary CB. Complications of fractional CO2 laser resurfacing: Four cases. Lasers Surg Med 2009;41:179-84.
[Google Scholar]
|
| 12. |
Alsousou J, Ali A, Willett K, Harrison P. The role of platelet-rich plasma in tissue regeneration. Platelets 2013;24:173-82.
[Google Scholar]
|
| 13. |
Lee JW, Kim BJ, Kim MN, Mun SK. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: A simultaneous split-face trial. Dermatol Surg 2011;37:931-8.
[Google Scholar]
|
| 14. |
Cervelli V, Nicoli F, Spallone D, Verardi S, Sorge R, Nicoli M, et al. Treatment of traumatic scars using fat grafts mixed with platelet-rich plasma, and resurfacing of skin with the 1540 nm nonablative laser. Clin Exp Dermatol 2012;37:55-61.
[Google Scholar]
|
| 15. |
Gawdat HI, Hegazy RA, Fawzy MM, Fathy M. Autologous platelet rich plasma: Topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg 2014;40:152-61.
[Google Scholar]
|
| 16. |
Nita AC, Orzan OA, Filipescu M, Jianu D. Fat graft, laser CO2 and platelet-rich-plasma synergy in scars treatment. J Med Life 2013;6:430-3.
[Google Scholar]
|
| 17. |
Nofal E, Helmy A, Nofal A, Alakad R, Nasr M. Platelet-rich plasma versus CROSS technique with 100% trichloroacetic acid versus combined skin needling and platelet rich plasma in the treatment of atrophic acne scars: A comparative study. Dermatol Surg 2014;40:864-73.
[Google Scholar]
|
| 18. |
Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: A milieu of bioactive factors. Arthroscopy 2012;28:429-39.
[Google Scholar]
|
| 19. |
Lacci KM, Dardik A. Platelet-rich plasma: Support for its use in wound healing. Yale J Biol Med 2010;83:1-9.
[Google Scholar]
|
| 20. |
Cho JW, Kim SA, Lee KS. Platelet-rich plasma induces increased expression of G1 cell cycle regulators, type I collagen, and matrix metalloproteinase-1 in human skin fibroblasts. Int J Mol Med 2012;29:32-6.
[Google Scholar]
|
| 21. |
Kim SA, Ryu HW, Lee KS, Cho JW. Application of platelet-rich plasma accelerates the wound healing process in acute and chronic ulcers through rapid migration and upregulation of cyclin A and CDK4 in HaCaT cells. Mol Med Rep 2013;7:476-80.
[Google Scholar]
|
| 22. |
Goodman GJ, Baron JA. Postacne scarring: A qualitative global scarring grading system. Dermatol Surg 2006;32:1458-66.
[Google Scholar]
|
| 23. |
Na JI, Choi JW, Choi HR, Jeong JB, Park KC, Youn SW, et al. Rapid healing and reduced erythema after ablative fractional carbon dioxide laser resurfacing combined with the application of autologous platelet-rich plasma. Dermatol Surg 2011;37:463-8.
[Google Scholar]
|
| 24. |
Shin MK, Lee JH, Lee SJ, Kim NI. Platelet-rich plasma combined with fractional laser therapy for skin rejuvenation. Dermatol Surg 2012;38:623-30.
[Google Scholar]
|
| 25. |
Marx RE. Platelet-rich plasma: Evidence to support its use. J Oral Maxillofac Surg 2004;62:489-96.
[Google Scholar]
|
| 26. |
Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: A review of biology and applications in plastic surgery. Plast Reconstr Surg 2006;118:147e-59e.
[Google Scholar]
|
| 27. |
Froum SJ, Wallace SS, Tarnow DP, Cho SC. Effect of platelet-rich plasma on bone growth and osseointegration in human maxillary sinus grafts: Three bilateral case reports. Int J Periodontics Restorative Dent 2002;22:45-53.
[Google Scholar]
|
| 28. |
Robiony M, Polini F, Costa F, Politi M. Osteogenesis distraction and platelet-rich plasma for bone restoration of the severely atrophic mandible: Preliminary results. J Oral Maxillofac Surg 2002;60:630-5.
[Google Scholar]
|
| 29. |
Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost 2004;91:4-15.
[Google Scholar]
|
| 30. |
May AE, Seizer P, Gawaz M. Platelets: Inflammatory firebugs of vascular walls. Arterioscler Thromb Vasc Biol 2008;28:s5-10.
[Google Scholar]
|
Fulltext Views
8,775
PDF downloads
3,640





