Translate this page into:
Efficacy of desonide 0.05% cream and lotion in steroid-responsive dermatoses in Indian patients: A post-marketing surveillance study
2 Galderma India Pvt. Ltd, Mumbai, India
Correspondence Address:
Percy H Sanjana
Galderma India Pvt. Ltd., 23 Steelmade Industrial Estate, 2nd Floor, Marol Village, Andheri (E), Mumbai - 400 059
India
| How to cite this article: Bhankharia DA, Sanjana PH. Efficacy of desonide 0.05% cream and lotion in steroid-responsive dermatoses in Indian patients: A post-marketing surveillance study. Indian J Dermatol Venereol Leprol 2004;70:288-291 |
Abstract
BACKGROUND: Desonide, a non-halogenated, low-potency topical steroid, is indicated in the treatment of steroid-responsive dermatoses. AIMS: A post-marketing surveillance study was conducted to evaluate the efficacy and safety of DesowenTM (Desonide 0.05%) cream and lotion in Indian patients for the treatment of steroid-responsive dermatoses of mild to moderate severity. METHODS: A 3-week, multicenter, open-label, non-comparative Phase IV trial involving 1789 patients from 229 centers across India was conducted between January and August 2003. RESULTS: Of the 1789 patients, 1483 patients completed the treatment as per protocol. At the end of the therapy, 98.5% of the patients showed an improvement in their dermatitis from baseline. More than half of the patients showed a greater than 75% improvement in their signs and symptoms. No adverse effects were noted in any of the patients. Both the lotion and cream were found to be equally effective. CONCLUSIONS: Desonide 0.05% is a safe and effective low-potency corticosteroid for the treatment of mild to moderate dermatoses in Indian patients. No clinically apparent side-effects were observed in infants less than 1 year of age.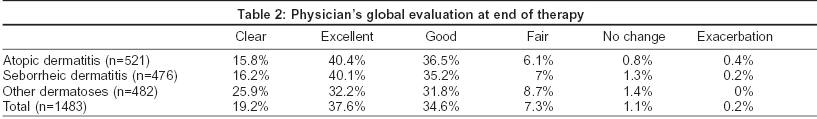


 |
 |
INTRODUCTION
Desonide is a non-halogenated, low-potency Group VI (McKenzie and Stoughton Classification) topical corticosteroid. It is a chemically modified hydrocortisone molecule suitable for the relief of pruritus and inflammation in corticosteroid-responsive dermatoses of mild to moderate severity as well as for the long-term and maintenance therapy of dry, scaly chronic dermatoses.[1] The cream formulation was introduced in India in 1997 and the lotion in 2001.
Like other topical steroids, desonide has anti-inflammatory, anti-pruritic and vasoconstrictive properties.[2],[3] Studies have shown that desonide is comparable in efficacy to other topical steroids such as fluocinolone acetonide, betamethasone valerate, hydrocortisone butyrate and triamcinolone acetonide, in the treatment of dermatitis.[4],[5],[6],[7],[8] Desonide has an anti-inflammatory action comparable to the fluorinated steroids in its class in spite of not having the fluorine atom in the 9th position.[9] Unlike most topical steroids, both desonide 0.05% cream and lotion were found to be equally potent and effective.[1]
Desonide 0.05% has been safely used in sensitive areas like the face.[10] Desonide cream 0.05% was found to have a dermal safety profile comparable to that of 1% hydrocortisone cream when used over an 8-week period, even when applied to normally thin skin (post-auricular areas). There were no signs of atrophy even after prolonged usage of either desonide or hydrocortisone.[11]
The aim of this multicenter, post-marketing surveillance study was to evaluate the efficacy and safety of desonide 0.05% cream and lotion in the treatment of Indian patients with steroid-responsive dermatoses of mild to moderate severity.
METHODS
In this open-label, non-comparative Phase IV clinical trial of desonide 0.05% cream and lotion, 1789 patients were enrolled from 229 centers across India between January and August 2003. Patients of any age and gender with a clinical diagnosis of any steroid-responsive dermatosis of mild to moderate variety with a clinical severity score between 1 and 11 were included in the trial. A written informed consent from patients or their guardians was obtained prior to study enrollment.
Each patient was evaluated for various signs and symptoms of inflammation including erythema, dryness or scaling, pruritus, excoriations, oozing or crusting. The above parameters were evaluated using a four-point scale (0 = none or clear; 1 = mild; 2 =moderate; 3 = severe). The pretreatment sum total of all evaluable parameters gave the total clinical severity score based on which the patients were classified as: mild dermatitis - total clinical severity score 1-5; moderate dermatitis -total clinical severity score 6-11; severe dermatitis - total clinical severity score 12-18.
Patients were excluded from the study if they had severe steroid-responsive dermatosis i.e. a clinical severity score of 12 or more or a known hypersensitivity to desonide cream or lotion. Pregnant or nursing women or patients who had received steroids (oral or topical) 1 week prior to study enrollment were also excluded.
Patients were advised to use either desonide cream or lotion 0.05% (DesowenTM) depending on the discretion of the investigator. Patients were asked to apply a thin layer to all the affected areas twice a day for 3 weeks. CetaphilTM cleansing lotion was used to cleanse the lesions in order to standardize the cleansing procedure. Any concomitant therapy with oral antibiotics or antihistamines was permitted as per the discretion of the investigator.
End of treatment scores were also noted using the same four-point scale. The physician′s global evaluation of the overall response to therapy was recorded as follows: clear (no signs or symptoms) - 100% improvement; marked improvement (excellent) - 75% or more improvement; definite improvement (good) - 50-74% improvement; minimal improvement (fair) - 25-50% improvement; no change - no detectable improvement from pretreatment evaluation; exacerbation - worsening of signs and symptoms
All adverse events were recorded in the case record forms. A single intention to treat analysis was done.
RESULTS
A total of 1789 patients were included in the study. Out of those, 306 patients did not follow up and 1483 patients completed the study as per the protocol. The data of these were analyzed. The male: female ratio was 1:1 and the age of the patients ranged from < 1 year to 36 years. Thirty-five per cent of the patients had atopic dermatitis, 32% of the patients had seborrheic dermatitis and 33% had other steroid-responsive dermatoses (including contact dermatitis, nummular eczema and other eczematous dermatoses).
In all the patients irrespective of their diagnosis and/or age group, there was on an average an 80% reduction in the total symptom severity score from the baseline level [Table - 1]. This is a statistically significant reduction in the mean scores as compared to baseline by Wilcoxon′s test. Reduction in the clinical severity score was comparable in all the age groups 79.6% for < 1 year group (n = 106), 82% for 1-5 years group (n = 257), and 81% for above 5 years group (n = 1120).
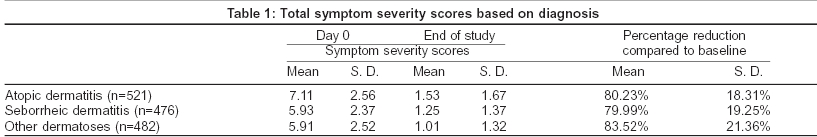
Both the formulations of desonide viz. cream and lotion were found to be equally effective in reducing the mean symptom severity scores for all the three groups of steroid responsive disorders. The percentage of patients in whom the baseline symptoms have been cleared completely is shown in [Figure - 1]. Results of the physician′s global assessment are shown in [Table - 2]. There was no difference in the physician′s global evaluation regarding the use of desonide cream or lotion across all steroid-responsive dermatoses. No adverse effects were reported after the use of desonide cream or lotion and no patients dropped out due to an adverse drug event.
DISCUSSION
Topical corticosteroids are the mainstay in the treatment of various eczemas such as atopic dermatitis, and seborrheic dermatitis, primarily because of their anti-inflammatory, anti-pruritic and vasoconstrictive actions.[10] Corticosteroids are classified into various categories according to their potency. Halogenation of the 9th position in the structure of hydrocortisone is known to significantly increase the potency of the steroid.[12]
Long-term use of potent and fluorinated topical steroids is known to produce local (skin atrophy, hypopigmentation, infections, telangiectasia, striae) and systemic (HPA axis suppression, Cushing′s syndrome, growth retardation in children) side-effects.[1] Frequent use of high-potency steroids on the facial skin can produce perioral dermatitis, persistent erythema, telangiectasia, skin atrophy, a papular-pustular eruption and a rosacea-like dermatitis as the number of steroid receptor sites are highest on the face.[13],[14],[15] However, the propensity of lower potency topical steroids to elicit such adverse effects is less than that of the more potent preparations.[1]
A low-potency steroid like desonide finds its usefulness in sensitive areas like the face, intertriginous folds, genitals and perianal areas. This is because there is an increased percutaneous absorption from these areas either due to a reduced thickness of the skin or due to natural occlusion. Enhanced penetration of topical steroids from these anatomical sites contributes to an increased incidence of local and systemic side-effects. In infants and children, low-potency corticosteroids are preferable as they are less likely to cause HPA axis suppression and growth retardation.
Low-potency steroids can be used as a step-down therapy in chronic dermatoses that require long-term therapy with topical corticosteroids for maintenance of remission. In one study, it was found that HPA suppression was rarely found in children or adolescents with moderate to severe atopic dermatitis who used mild or moderately potent topical corticosteroids for several years. However, HPA axis suppression was common in those receiving potent topical steroid preparations.[16]
In an Australian study desonide 0.05% lotion was found to be highly efficacious in reducing the severity of atopic and seborrheic facial dermatitis. DesowenTM lotion, a moisturizing, alcohol-free, non-comedogenic preparation, was found by patients to be cosmetically acceptable with 95% of patients stating that they would use the lotion again.[10]
Of the 1483 patients who completed this study, 98.6% showed a global improvement in their disease. In 91.4% there was global improvement of greater than 50% in their disease from baseline. Complete clearance of all lesions was observed in 19% with another 38% demonstrating excellent improvement (> 75%) in their disease. Lesions remained unchanged or were exacerbated in only 1.16% and 0.21% of patients respectively. These data conform to the results obtained in other randomized trials.[1],[4],[8],[17]
The similar potency of desonide cream and lotion was correlated clinically with both formulations reducing symptom severity scores equally. Desonide was found to be safe and efficacious in children < 1 year of age. Hence desonide may be considered as an efficacious and safe option in the treatment of infants with steroid-responsive dermatoses.
This study has clearly demonstrated the efficacy and safety of desonide cream in Indian patients and thus we can conclude that desonide cream/lotion 0.05% (DesowenTM) is an effective and safe modality of treatment in Indian patients suffering from mild to moderate steroid-responsive dermatoses.
| 1. |
Prawer SE, Katz HI, Herndon JH, Baker MD, Cheney T. A Controlled, bilateral, comparative evaluation of 0.05% desonide lotion and desonide cream in eczematous dermatitis. Curr Ther Res 1993;53:607-13.
[Google Scholar]
|
| 2. |
Chren MM, Bickers DR. Dermatological Pharmacology. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. Goodman and Gilman's -The pharmacological basis of therapeutics. New York: Pergamon Press; 1990. p. 1572-92.
[Google Scholar]
|
| 3. |
Bangham AD, Standish MM, Weismann G. The action of steroids and streptolysin S on the permeability of phospholipid structures to cations. J Mol Biol 1963;13:253-9.
[Google Scholar]
|
| 4. |
Barsky S. Clinical comparison of desonide cream with fluocinonide cream in steroid responsive dermatologic disorders. Cutis 1976;18:826-30.
[Google Scholar]
|
| 5. |
Bluefarb SM. Clinical comparison of desonide cream with betamethasone valerate cream. A double blind randomized study. Int J Dermatol 1972;11:73-6.
[Google Scholar]
|
| 6. |
Henrijean A, Lapiere CM. Clinical evaluation of desonide cream. Curr Ther Res 1983;33:775-81.
[Google Scholar]
|
| 7. |
Donsky HJ. A comparative double-blind randomized clinical study of a new, non-fluorinated topical corticosteroid. Cutis 1972;46-8.
[Google Scholar]
|
| 8. |
Gentry WC Jr, Rosenberg EW, Goltz RW. A clinical evaluation of 0.05% desonide cream - a new nonfluorinated topical corticosteroid. Arch Dermatol 1973;107:870-1.
[Google Scholar]
|
| 9. |
Reque PG. The treatment of common dermatoses with topically applied 0.05% desonide cream. J Med Assoc State Ala 1972;42:25-7.
[Google Scholar]
|
| 10. |
Freeman S, Howard A, Foley P, Rosen R, Wood G, See J, et al. Efficacy, cutaneous tolerance and cosmetic acceptability of desonide 0.05% lotion (Desowen) versus vehicle in the short - term treatment of facial atopic and seborrheic dermatitis. Australas J Dermatol 2002;45:186-9.
[Google Scholar]
|
| 11. |
Cornell RC, Baker MD. Dermal safety comparison of 0.05% desonide cream and 1% hydrocortisone cream. Curr Ther Res 1993;53:356-9.
[Google Scholar]
|
| 12. |
Smith EB, Gregory JF, Bartruff JK. Desonide: A potent non-fluorinated topical steroid, vasoconstriction assay and clinical trial. South Med J 1973;66:325-9.
[Google Scholar]
|
| 13. |
Leyden JJ, Thew M, Kligman AM. Steroid rosacea. Arch Dermatol 1974;110:619-22.
[Google Scholar]
|
| 14. |
Sneddon I. Perioral dermatitis. Br J Dermatol 1972;82:430.
[Google Scholar]
|
| 15. |
Sneddon I. Adverse effects of topical fluorinated corticosteroids in rosacea. Br Med J 1969;1:671-3.
[Google Scholar]
|
| 16. |
Ellison JA, Patel L, Ray DW, David TJ, Clayton PE. Hypothalamic pituitary-adrenal function and glucocorticoid sensitivity in atopic dermatitis. Pediatrics 2000;105:794-9.
[Google Scholar]
|
| 17. |
Jorizzo J, Levy M, Lucky A, Shavin J, Goldberg G, Dunlap F, et al. Multicenter trial for long term safety and efficacy comparison of 0.05% desonide and 1% hydrocortisone ointments in the treatment of atopic dermatitis in pediatric patients. J Am Acad Dermatol 1995;33:74-7.
[Google Scholar]
|
Fulltext Views
4,410
PDF downloads
535





