Translate this page into:
Efficacy of platelet-rich plasma in Asians with androgenetic alopecia: A randomized controlled trial
Corresponding author: Senior Consultant, Sai Yee Chuah, Department of Dermatology, National Skin Centre, 1 Mandalay Rd, Singapore. sychuah@nsc.com.sg
-
Received: ,
Accepted: ,
How to cite this article: Chuah SY, Tan CH, Wang ECE, Tan KT, Chan RKW, Zhao X, et al. Efficacy of platelet-rich plasma in Asians with androgenetic alopecia: A randomized controlled trial. Indian J Dermatol Venereol Leprol 2023;89:135-8.
Sir,
Autologous platelet-rich plasma offers promise as a therapeutic option in androgenetic alopecia. Platelet-rich plasma is a concentrated suspension of platelets harvested from a patient’s venous blood. To date, there are only five prospective cohort studies and one randomized controlled trial in Asians.1 Thus, we conducted this 24-week, double-blind, placebo-controlled trial to investigate the impact of platelet-rich plasma in Asian patients of diverse ethnicities with androgenetic alopecia in Singapore. The study protocol was assessed and approved by the Ethics committee from the National Healthcare Group Institutional Review Board. The trial is registered under the Health Services Authority of Singapore and was granted approval for conduct under the Medicines (Clinical Trials) Regulations, 1978.
Platelet-rich plasma was prepared using the platelet-rich plasma RegenKit® (NeoAsia (S) Pte Ltd).2 Every participant received platelet-rich plasma injections, randomized to one half of the scalp, with the other half injected with normal saline (placebo treatment). A total of four treatment cycles every three weeks were carried out (Visits 1–4). They were reviewed at 12 and 24 weeks (Visit 5 and 6) for hair analysis with Folliscope® by LeedM Corp in Seoul, scalp photography of the vertex and occipital areas for comparison by three blinded dermatologists, and completion of a validated hair growth questionnaire.
Fifty patients (32 male, 18 female) of good general health completed the study. The clinical characteristics of our study participants are shown in Table 1. The results on hair density and hair diameter are shown in Table 2 and Figure 1.
| N (%) | |||
|---|---|---|---|
| All (n = 55) |
Male (n = 34) |
Female (n = 21) |
|
| Completion of all study visits | 50 (90.9%) | 32 (94.1%) | 18 (85.7%) |
| Withdrawn | 5 (9.1%) | 2 (5.9%) | 3 (14.3%) |
| Withdrawn at week 12 | 3 (5.5%) | 1 (2.9%) | 2 (9.5%) |
| Withdrawn at week 24 | 2 (3.6%) | 1 (2.9%) | 1 (4.8%) |
| Age | |||
| mean ± standard deviation (SD) | 38.7 ± 10.5 | 36.5 ± 8.8 | 42.2 ± 12.2 |
| median (min, max) | 38 (23, 70) | 37 (23, 58) | 39 (23, 70) |
| Race | |||
| Chinese | 41 (74.6%) | 23 (67.7%) | 18 (85.7%) |
| Malay | 8 (14.5%) | 6 (17.6%) | 2 (9.5%) |
| Indian | 4 (7.3%) | 3 (8.8%) | 1 (4.8%) |
| Filipino | 2 (3.6%) | 2 (5.9%) | 0 (0.0%) |
| Gender | |||
| Male | 34 (61.8%) | 34 (100.0%) | – |
| Female | 21 (38.2%) | – | 21 (100.0%) |
| Patient Norwood-Hamilton level | |||
| III | 14 (41.2%) | 14 (41.2%) | – |
| IV | 13 (38.2%) | 13 (38.2%) | – |
| V | 5 (14.7%) | 5 (14.7%) | – |
| VI | 2 (5.9%) | 2 (5.9%) | – |
| Patient Ludwig level | |||
| II | 16 (29.6%) | – | 16 (76.2%) |
| III | 5 (9.3%) | – | 5 (23.8%) |
| Age of onset of AGA | |||
| mean ± standard deviation (SD) | 31.3 ± 10.7 | 30.2 ± 7.3 | 33.1 ± 14.6 |
| median (min, max) | 29 (15, 65) | 29 (15, 49) | 26 (16, 65) |
| Duration of AGA | |||
| mean ± SD | 7.3 ± 5.5 | 6.2 ± 5.2 | 9.1 ± 5.7 |
| median (min, max) | 5 (1, 20) | 4.5 (1, 20) | 8 (2, 20) |
SD: Standard deviation, AGA: androgenetic alopecia
| Placebo | PRP treatment | Placebo vs. PRP | |||
|---|---|---|---|---|---|
| Mean (95% CI) | P* | Mean (95% CI) | P* | P | |
| All participants | |||||
| Hair density (number of hair shafts/cm2) | |||||
| Baseline | 128.8 (121.4, 136.2) | – | 126.6 (119.1, 134.2) | – | 0.48 |
| 12 weeks - baseline | 4.6 (-3.4, 12.6) | 0.16 | 12.7 (5.8, 19.7) | 0.001 | 0.10 |
| 24 weeks - baseline | 10.0 (1.0, 19.0) | 0.02 | 14.1 (7.9, 20.3) | <0.001 | 0.20 |
| Hair diameter (μm) | |||||
| Baseline | 66.9 (63.0, 70.8) | 65.5 (62.0, 69.0) | 0.41 | ||
| 12 weeks - baseline | –2.8 (–5.9, 0.2) | 0.07 | –1.1 (–3.6, 1.4) | 0.62 | 0.35 |
| 24 weeks - baseline | –3.9 (–7.3, –0.5) | 0.03 | 0.1 (-2.6, 2.9) | 0.57 | 0.03 |
| Males | |||||
| Hair density (number of hair shafts/cm2) | |||||
| Baseline | 137.6 (128.7, 146.4) | – | 134.9 (124.4, 145.3) | – | 0.33 |
| 12 weeks - baseline | 4.8 (-3.8, 13.5) | 0.25 | 16.7 (7.4, 26.1) | 0.001 | 0.03 |
| 24 weeks - baseline | 11.8 (0.6, 23.0) | 0.08 | 14.6 (5.6, 23.6) | 0.002 | 0.27 |
| Hair diameter (μm) | |||||
| Baseline | 64.6 (59.6, 69.6) | 63.2 (58.8, 67.7) | 0.69 | ||
| 12 weeks - baseline | –4.0 (-8.1, 0.1) | 0.05 | –3.1 (–6.4, 0.3) | 0.14 | 0.80 |
| 24 weeks - baseline | –5.9 (–10.4, –1.4) | 0.01 | –1.6 (–5.4, 2.2) | 0.56 | 0.06 |
| Females | |||||
| Hair density (number of hair shafts/cm2) | |||||
| Baseline | 114.6 (103.8, 125.3) | – | 113.2 (105.7, 120.7) | – | 0.90 |
| 12 weeks - baseline | 4.3 (-12.0, 20.5) | 0.44 | 5.8 (-3.4, 15.0) | 0.21 | 0.89 |
| 24 weeks - baseline | 6.7 (-8.7, 22.1) | 0.15 | 13.2 (6.5, 19.9) | 0.002 | 0.57 |
| Hair diameter (μm) | |||||
| Baseline | 70.7 (64.4, 76.9) | – | 69.1 (63.6, 74.6) | – | 0.39 |
| 12 weeks - baseline | –0.8 (-5.4, 3.7) | 0.63 | 2.3 (-1.2, 5.8) | 0.18 | 0.25 |
| 24 weeks - baseline | –0.3 (–5.2, 4.7) | 0.76 | 3.2 (-0.1, 6.5) | 0.03 | 0.30 |
*P: P-value was obtained from the Wilcoxon signed-rank test for comparing the change in hair density or hair diameter from baseline to 12 weeks or 24 weeks vs. no change, PRP: Platelet-rich plasma
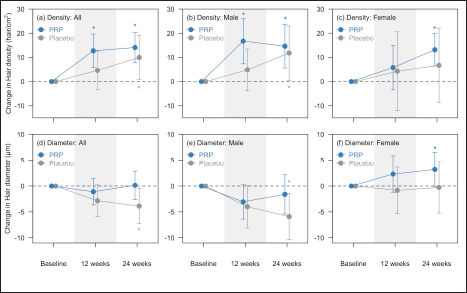
- Summary of change in hair density and hair diameter by treatment and gender at baseline, 12 weeks, and 24 weeks. Top panel shows the results for hair density. In male participants, there was significant increase on the PRP-treated side at both 12 weeks and 24 weeks, and significant increase on the saline-treated side (control) only at 24 weeks. In female participants, there was significant increase only on the PRP-treated side at 24 weeks. For all participants, there was significant increase at 12 and 24 weeks on PRP-treated side, and significant increase on the saline-treated side (control) only at 24 weeks. Bottom panel shows the results for hair diameter. In male participants, decrease in hair diameter was observed but only significant on the saline-treated side (control) at 24 weeks. In female participants, there was absolute increase in hair diameter on PRP-treated side at 12 and 24 weeks but did not reach statistical significance. For all participants, there was significant decrease in hair diameter on the saline-treated side (control) at 24 weeks. Points represent the means with whiskers indicating the 95% confidence intervals. Asterisks indicate significant difference compared with baseline within each treatment group based on bivariable analysis.
Overall, platelet-rich plasma injections resulted in an increase in hair density as early as in the first three months of treatment amongst male participants. Interestingly, there was no significant change in hair diameter with platelet-rich plasma but a progressive decrease in hair diameter with placebo treatment in our study. In androgenetic alopecia, hair miniaturization is characteristic and hence, hair diameter is expected to decrease over time. The maintenance of hair diameter on platelet-rich plasma-treated scalp suggests that platelet-rich plasma could provide the additional benefit of halting the reduction in hair shaft diameter. This effect was also reported in one Korean male patient with androgenetic alopecia.3 Moreover, multivariate analysis [Figure 2] revealed a trend towards progressive reduction in hair diameter in men with more advanced hair loss. This was also observed in a prospective cohort study done in China,4 suggesting that platelet-rich plasma should be initiated early for better efficacy.

- Regression coefficients (β) derived from multivariable linear mixed effect model for hair density (a, c) and hair diameter (b, d) by gender. Points are regression coefficients (β1) with whiskers indicating the 95% confidence intervals. If the 95% confidence interval does not cover zero, the corresponding regression coefficient is statistically significant
In our study, there was a trend of increased hair density in women treated with platelet-rich plasma [Figure 2] but this was not statistically significant when compared to placebo. The inability to reach significant change could be due to the lack of female participants. The sample size required for this study was calculated to assess the efficacy from baseline to 24 weeks within each treatment arm, particularly in men. The efficacy analysis for females and all participants were secondary and mainly exploratory. The data on the efficacy of platelet-rich plasma in women is still lacking, and future, larger controlled trials are required to determine if platelet-rich plasma is an effective modality for female pattern hair loss.
At 24 weeks, there was also a significant increase in hair density with saline injections. This was also seen in a recent study by Shapiro et al.5 We postulate that this effect may be explained by the release of growth factors and cytokines as part of the wound healing process secondary to scalp injections, similar to microneedling.
When comparing the scalp photographs, our clinicians found significant hair growth at both 12 and 24 weeks in all participants. Similarly, the self-administered survey revealed patients found the treatments effective at slowing down the hair loss process or aiding in hair growth. There was no significant difference in attitudes and satisfaction at 12 and 24 weeks, despite objective improvements at 12 weeks in hair density. This inconsistency may be attributed to a placebo effect, or the hair parameters measured may not be indicative of patient-perceived improvement. Platelet-rich plasma injections were also well-tolerated and, interestingly, yielded lower pain scores when compared with saline injections.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Platelet-rich plasma and stem cells for hair growth: A review of the literature. Aesthet Surg J. 2020;40:NP177-88.
- [CrossRef] [PubMed] [Google Scholar]
- Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-9.
- [CrossRef] [PubMed] [Google Scholar]
- Letter: Platelet-rich plasma for treating male pattern baldness. Dermatol Surg. 2012;38:2042-44.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of platelet-rich plasma for treating androgenetic alopecia of varying grades. Clin Drug Investig. 2019;39:865-72.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of platelet-rich plasma as a treatment for androgenetic alopecia: A randomized controlled trial. J Am Acad Dermatol. 2020;83:1298-303.
- [CrossRef] [Google Scholar]





