Translate this page into:
Efficacy of topical tofacitinib 2% cream in the treatment of nail lichen planus
Corresponding author: Dr. Matilde Iorizzo, Private Dermatology Practice, Bellinzona/Lugano, Switzerland. matildeiorizzo@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Iorizzo M. Efficacy of topical tofacitinib 2% cream in the treatment of nail lichen planus. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1443_2024
Dear Editor,
Lichen planus (LP) is a benign inflammatory disease of unknown aetiology and has a chronic course that affects the skin, mucosae, scalp, and nails.1 It is usually asymptomatic, but the frequent severe nail abnormalities and the scarring potential can have a high impact on the quality of life of affected patients. Moreover, treatment is notoriously difficult further impacting on the patient’s lifestyle. An expert consensus has been published to guide clinicians; however, the disease still has the potential to recur and often undermines many of the proposed treatments.2 Steroids, both intralesional and systemic, have been found to be safe and effective as a first-line treatment, but many physicians still refuse to administer intralesional injections or are reluctant to prescribe systemic medications in general when only nails are affected.2 Nails constitute a small portion of the body surface area, and the potential adverse effects associated with systemic medications may often outweigh the benefits of prescribing them. Research of new, effective treatments with limited or no side effects is always welcome.
Recent developments in understanding the immunopathology of LP have led to the exploration of Janus kinase (JAK) inhibitors as a potential treatment. Oral tofacitinib and oral baricitinib have been used with success in nail LP and also in many other clinical presentations.3,4 Due to the high monthly cost and the off-label nature of their use, which is not covered by the healthcare system, the potential for topical administration has been explored. This approach aims to determine whether topical application can provide sufficient efficacy while minimizing systemic side effects.
Three men aged, 65, 51 and 59 years respectively and affected by recalcitrant, biopsy-proven LP limited to few fingernails were asked to apply a compounded cream made of 2% tofacitinib powder, phenoxyethanol, and cream base DAC twice daily (30 g tube with a shelf-life of 6 months) in the periungual area and on the nail plate. The compound was used because tofacitinib is not available as a marketed product in Europe. A cream was chosen over an ointment hoping to find more compliance with equal effectiveness. All patients agreed to sign a written informed consent for publication of their case details. The ethical principles for human studies as outlined in the Declaration of Helsinki were followed and attested.
The patients were otherwise healthy and no potential triggers of nail LP were detected in their medical history including a manual working condition or hobbies (to rule out koebnerisation) and smoking. All of them were previously treated with intramatricial steroid injections (2.5–5 mg/mL triamcinolone acetonide once a month for 4–6 months, 0.2 mL per nail unit per session) with moderate results and recurrences soon after stopping the treatment. None of them accepted a systemic treatment for fear of side effects. A wash-out period of 6 months was requested before starting 2% of the tofacitinib cream. This timeframe was selected based on the nail plate growth rate (3 mm/month for fingernails and 1.5 mm/month for toenails), ensuring adequate nail plate regeneration for evaluating treatment outcomes. Patients were instructed not to wash their hands for at least 4 hours after the application of the cream. After 12 weeks of continuous treatment, all patients showed significant improvement of their condition. See Figures 1–3 reporting the before and after nail lichen planus severity index (NALSI) scores.5 Patients reported good compliance with no local and systemic adverse events. Routine laboratory tests required at baseline and during treatment with oral JAK inhibitors treatment were done and no abnormality was reported. Patients are still under treatment–risk of recurrence after stopping the cream has made them reluctant to stop.
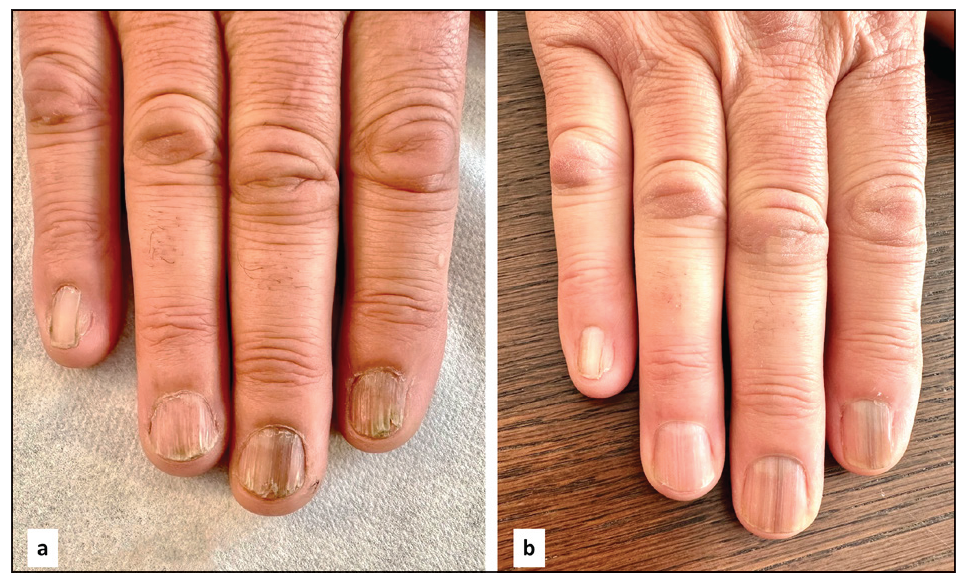
- A 65-year-old man before (a) and after (b) 3 months of treatment. NALSI, calculated from the fifth to the second fingernail, is 4-I/8/8/8 at baseline and it is reduced to 0-I/0/4/2 after 3 months. Notably, pterygium and longitudinal melanonychia seem to be resistant to treatment.
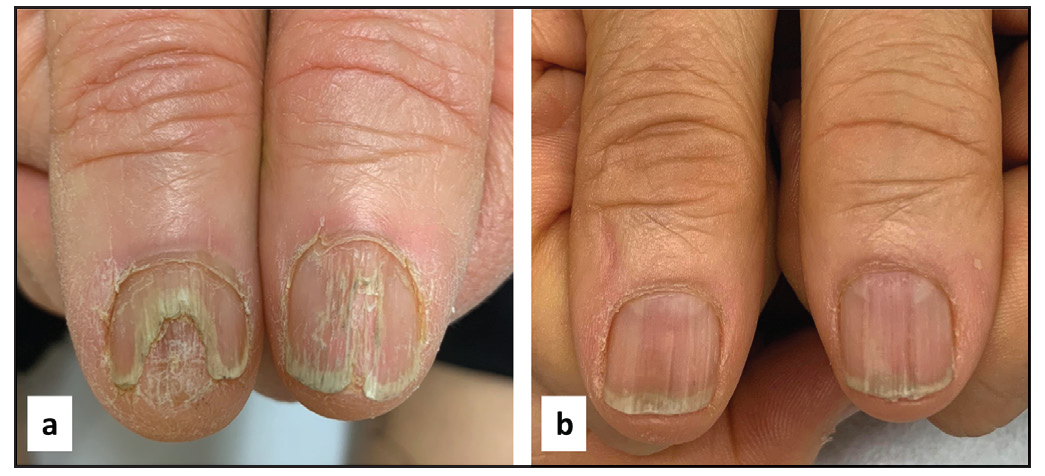
- A 51-year-old man before (a) and after (b) 3 months of treatment. NALSI, calculated from the right to the left patient’s nail, is 10/8 at baseline and it is reduced to 4/4 after 3 months.
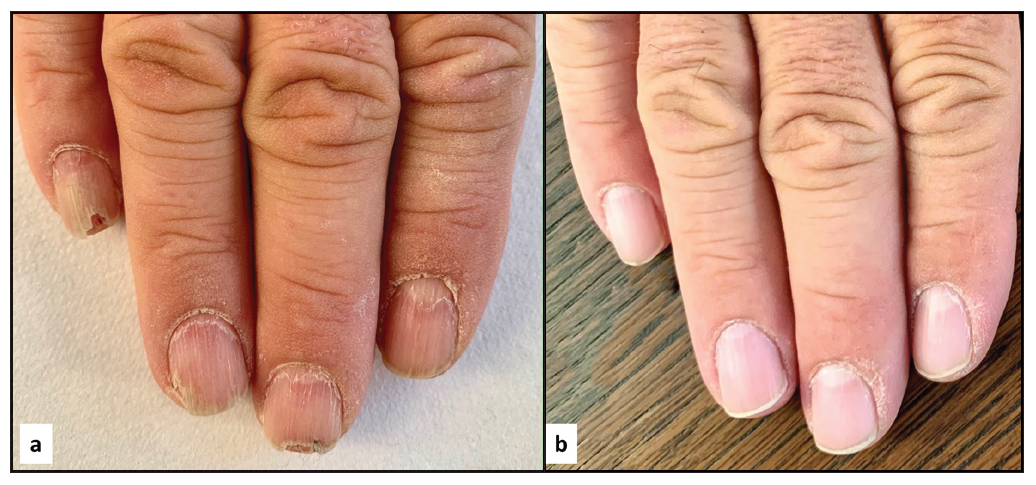
- A 59-year-old before (a) and after (b) 3 months of treatment. NALSI, calculated from the fifth to the second fingernail, is 8/6/6/4 at baseline and it is reduced to 0/0/0/0 after 3 months.
Preliminary data suggest that topical tofacitinib may be a potential treatment for nail lichen planus (LP). This medication has successfully controlled LP localised to other body areas, prompting exploration of its efficacy for nail involvement as well.6
Tofacitinib acts by inhibiting the JAK1/3–STAT pathways with the consequent suppression of the interferon-γ-mediated signal, one of the main components of the LP inflammatory infiltrate responsible for dermo-epidermal junction keratinocyte apoptosis and subsequent tissue damage. JAK1–STAT1 is, in particular, the pathway upregulated in LP according to recent findings.7
This study has been limited to three patients due to the high cost of the compounded cream. Further studies on bigger groups are necessary to confirm these results and establish the effectiveness and long-term safety in more severe cases or in children. Studies on penetration are also necessary in order to better understand the differences between the cream and the ointment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Nail lichen planus: A review of clinical presentation, diagnosis and therapy. Ann Dermatol Venereol. 2022;149:150-64.
- [CrossRef] [PubMed] [Google Scholar]
- Isolated nail lichen planus: An expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-23.
- [CrossRef] [PubMed] [Google Scholar]
- Tofacitinib as treatment for nail lichen planus associated with alopecia universalis. JAMA Dermatol. 2021;157:352-3.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of severe nail lichen planus with baricitinib. JAMA Dermatol. 2022;158:107-8.
- [CrossRef] [PubMed] [Google Scholar]
- Nail Lichen Planus Severity Index (NALSI): A novel severity score for nail lichen planus. Indian Dermatol Online J. 2022;13:680-1.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of resistant lichen planopilaris with topical tofacitinib monotherapy. Ann Dermatol Venereol. 2024;151:103303.
- [CrossRef] [PubMed] [Google Scholar]
- The inflammation in cutaneous lichen planus is dominated by IFN-ϒ and IL-21-A basis for therapeutic JAK1 inhibition. Exp Dermatol. 2021;30:262-70.
- [CrossRef] [PubMed] [Google Scholar]





