Translate this page into:
Endocrine mucin-producing sweat gland carcinoma: Reappraisal of patient demographics and tumour immunophenotypes
Corresponding author: Dr. Chiau-Sheng Jang, Department of Dermatology, Kaohsiung Veterans General Hospital, No. 386, Dazhong 1st Rd., Zuoying Dist., Kaohsiung City, Taiwan. macemars@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chuang I, Jang C. Endocrine mucin-producing sweat gland carcinoma: Reappraisal of patient demographics and tumour immunophenotypes. Indian J Dermatol Venereol Leprol 2022;88:544-7.
Sir,
Endocrine mucin-producing sweat gland carcinoma is a recently classified, low-grade skin adnexal neoplasm harbouring a neuroendocrine phenotype both morphologically and immunohistochemically. It is considered as a cutaneous analogue of solid papillary carcinoma in the breast, showing features of female predilection and estrogen receptor/progesterone receptor immunoreactivity.1 We herein describe three such cases and share some interesting findings on this peculiar entity.
The first case was a 68-year-old man with a slow-growing, skin-coloured nodule, 6 x 5 mm in size, at the right infraocular region. Microscopic examination showed a dermal-based, multinodular tumour featuring solid, cribriform and vague papillary architectures, with retraction clefts from the fibrous stroma [Figure 1a]. The neoplastic cells displayed round to ovoid, and occasionally spindled nuclei, with stippled chromatins and focal rosette-like arrangements [Figure 1b]. A few mitotic figures were found and no tumour necrosis was discernible. There were foci of extracellular mucin deposition, but intracytoplasmic mucin vacuoles were less conspicuous. Robust nuclear immunoexpressions of estrogen receptor, progesterone receptor, insulinoma-associated protein 1 and anti-c-Myb antibody were evident in more than 95% of tumour cells [Figure 1c - e], but only 10% of which stained positively and weakly for synaptophysin. Additionally, staining of androgen receptor was also identified, patchy and yet distinct, in 30% of tumour cells [Figure 1f].
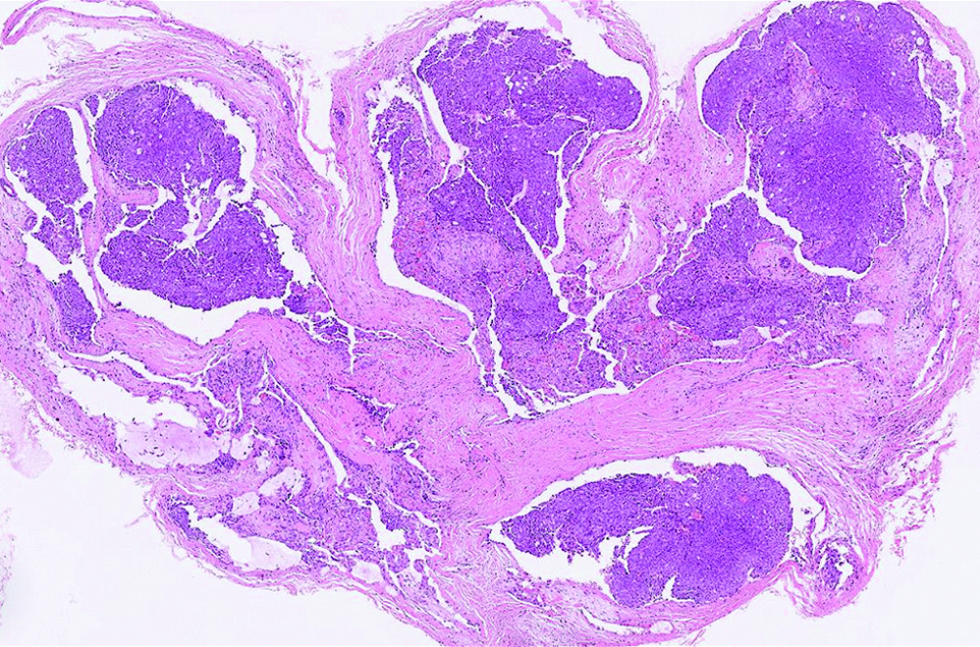
- The multinodular tumour with solid, cribriform and vague papillary architectures, with peripheral artifactual spaces in the first case (hematoxylin and eosin, ×100)
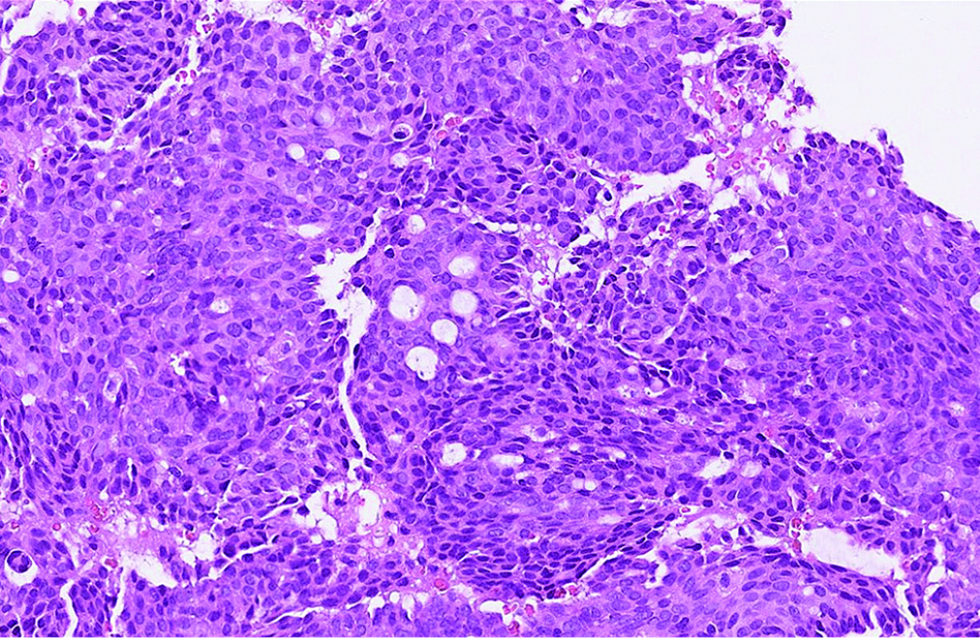
- The tumour cells with round, ovoid to spindled nuclei, with stippled chromatins and occasional rosette-like arrangements in the first case (hematoxylin and eosin, ×600)
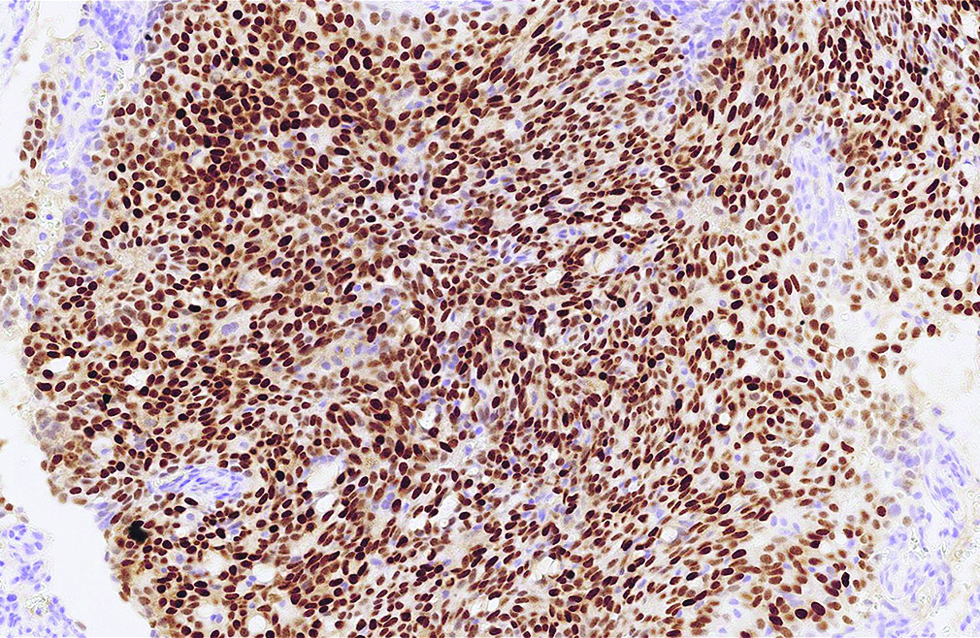
- Crisp nuclear expression of insulinoma-associated protein 1 was detected in more than 95% of tumour cells in the first case (immunohistochemistry, ×500)
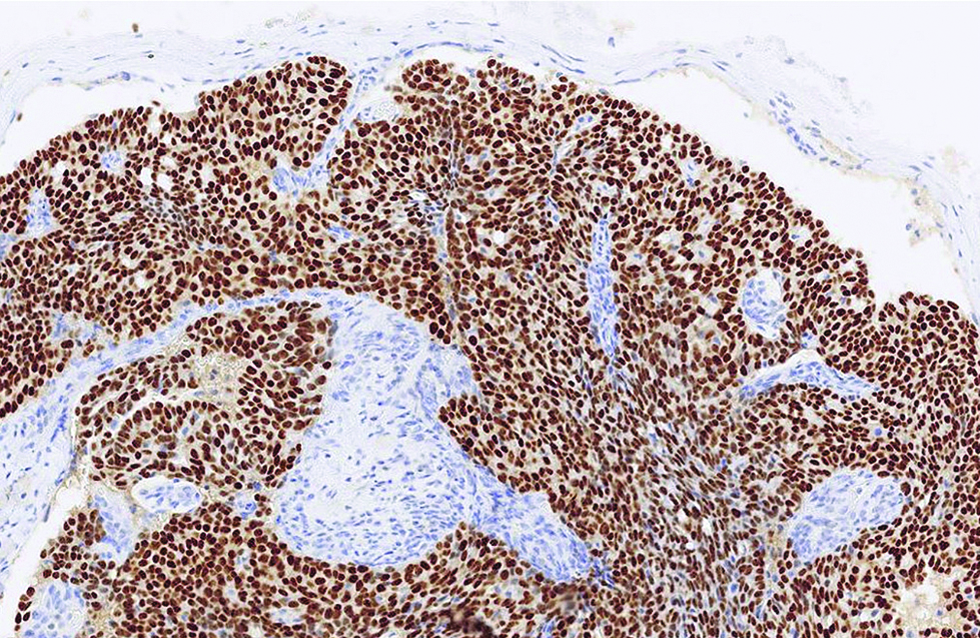
- The tumour with strong and diffuse immunoreactivity to estrogen receptor in the first case (immunohistochemistry, ×400)
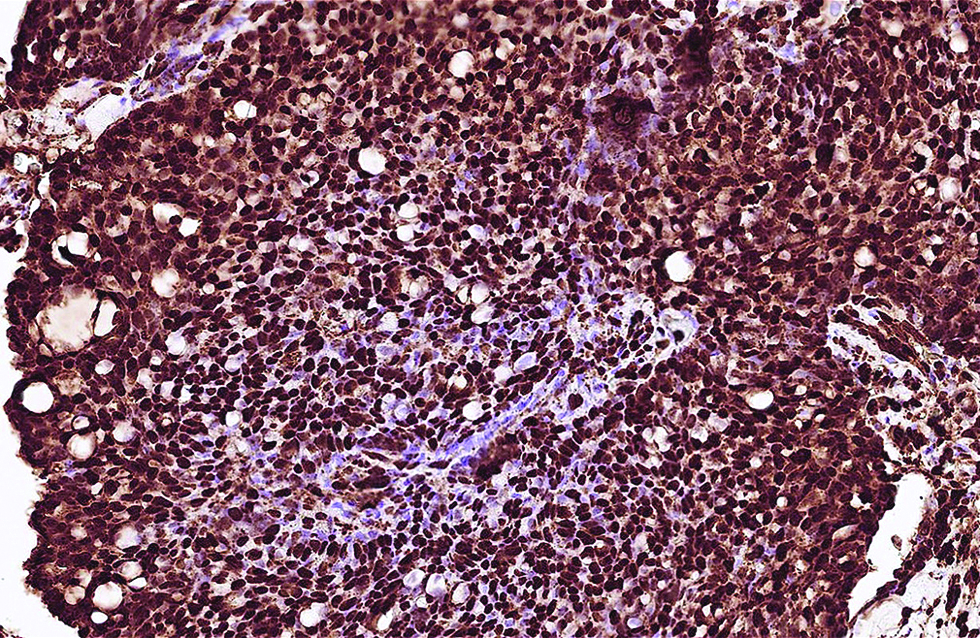
- Robust positivity of Myb antibody was identified in the first case (immunohistochemistry, ×600)
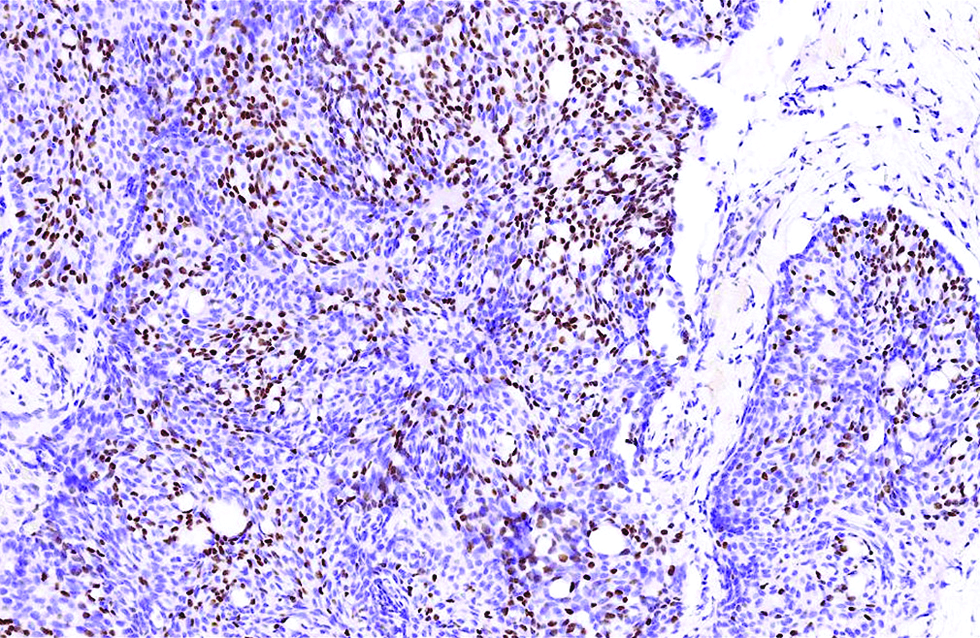
- Staining of androgen receptor was relatively patchy but distinct, in 30% of tumour cells in the first case (immunohistochemistry, ×400)
Different from the aforementioned histological pictures, the tumour of the second case was predominantly cystic, forming an 8 x 6 mm nodule at the left lower eyelid of a 52-year-old man. The cysts were filled with bluish mucin, and the tumour cells proliferated as papillary and micropapillary epithelial projections containing hyalinized fibrovascular cores and stroma. Cribriform and even acinar patterns were focally seen at the tumour periphery, a finding worrisome of invasion [Figure 2a]. The tumour cells appeared plump and columnar in shape, harbouring amphophilic to pinkish cytoplasm with occasional apical snouts [Figure 2b]. Immunohistochemical study of estrogen receptor, progesterone receptor and Myb antibody yielded a similarly strong and diffuse staining pattern, while a small portion of tumour cells was immunoreactive to insulinoma-associated protein 1 (15%) and synaptophysin (<5%). Androgen receptor stained 70% of tumour cells and spared the myoepithelial cells [Figure 2c - f].
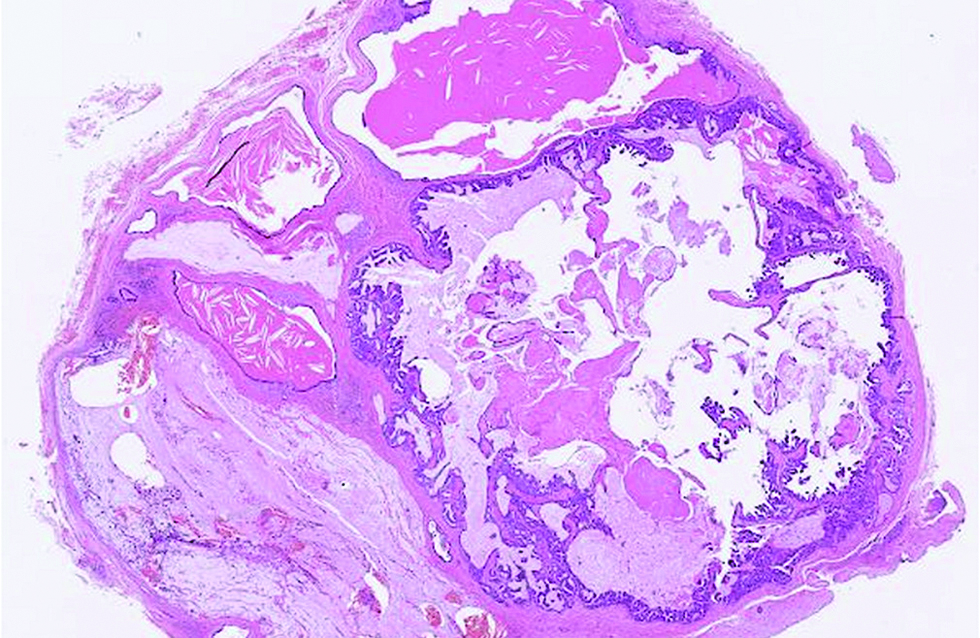
- The tumour predominantly cystic and filled with mucin in the second case (hematoxylin and eosin, ×25)
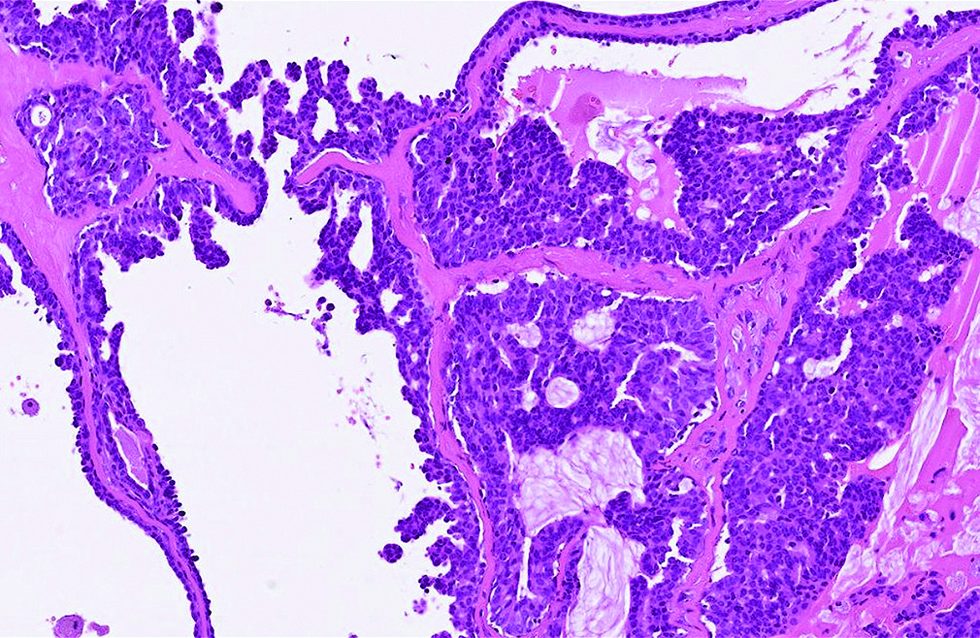
- Tumor cells harboured pinkish cytoplasm with occasional apical snouts, and proliferated as papillary and micropapillary projections separated by hyalinized stroma in the second case (hematoxylin and eosin, ×400)
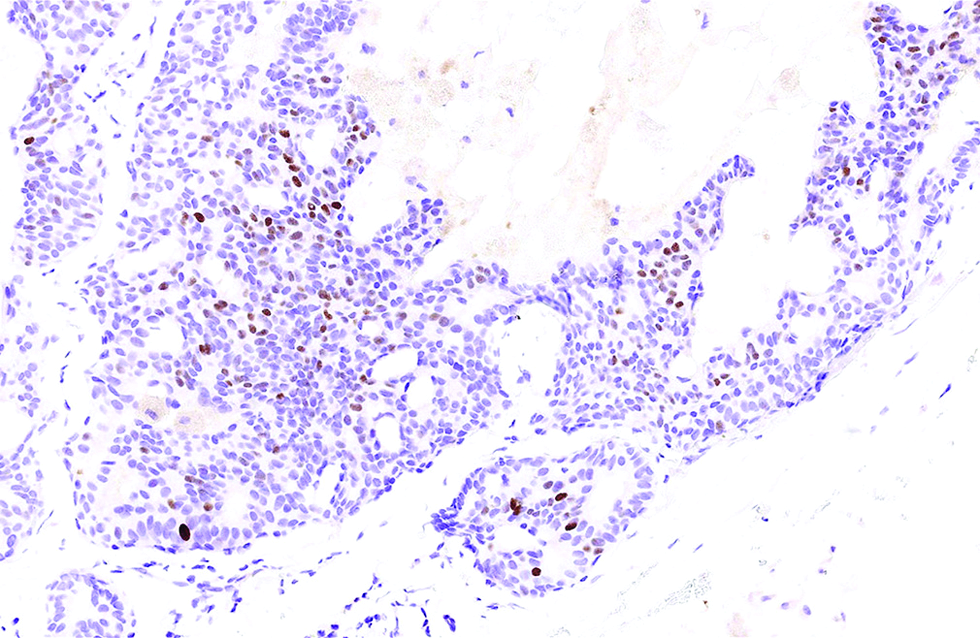
- A small portion (15%) of tumour cells immunoreactive to insulinoma-associated protein 1 in the second case (immunohistochemistry, ×500)
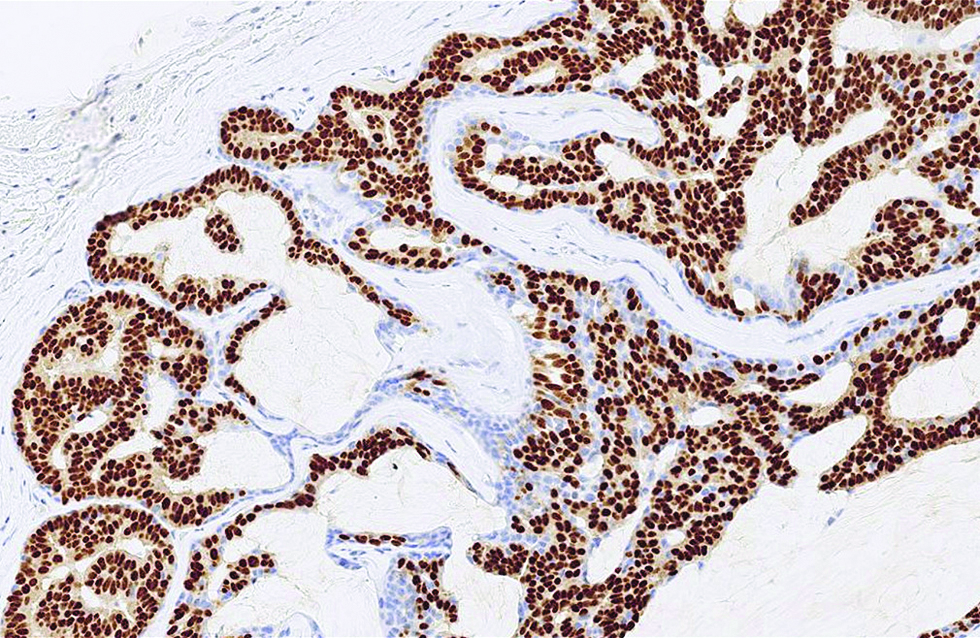
- The staining pattern of estrogen receptor strong and diffuse in the second case (immunohistochemistry, ×400)
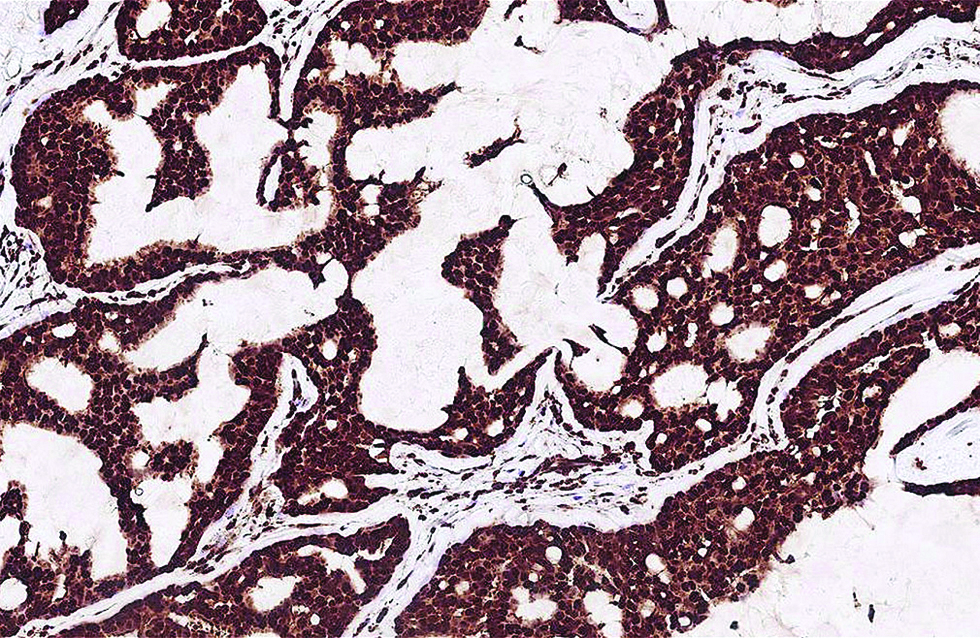
- Distinct nuclear expression of Myb antibody in more than 95% of tumour cells in the second case (immunohistochemistry, ×400)
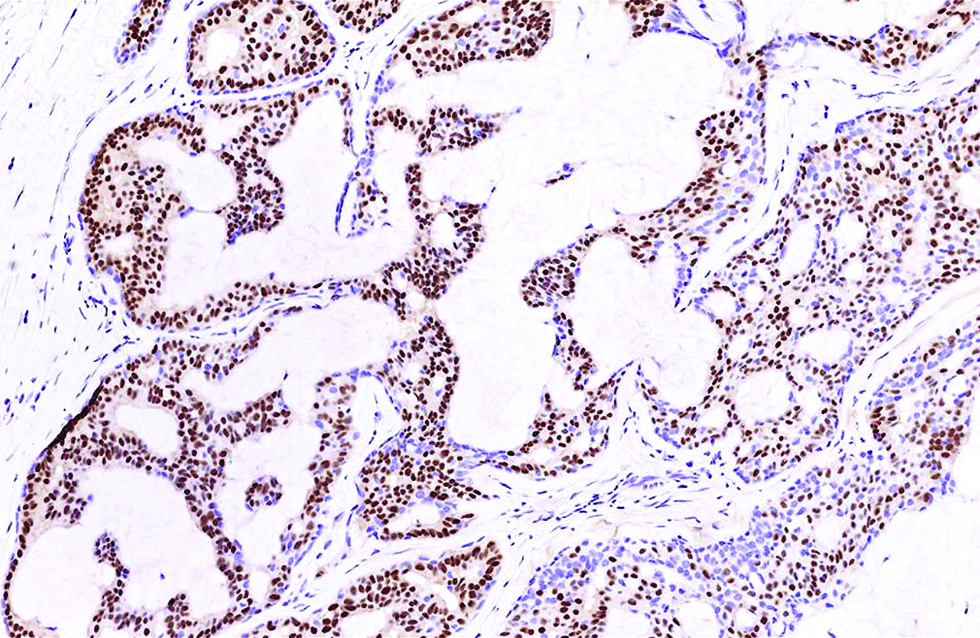
- Androgen receptor stained about 70% of tumour cells and spared the myoepithelial cells in the second case (immunohistochemistry, ×400)
The third case was a 54-year-old man with a skin-coloured, non-tender and gradually enlarging nodule for five months, 11 x 9 mm in size, at the lateral canthal area of the left eye [Figure 3]. The histopathologic feature and the immunohistochemical phenotype were similar to that of the first case, showing a predominant solid-papillary arrangement with distinct nuclear expressions of estrogen receptor (95%), progesterone receptor (95%), androgen receptor (90%), insulinoma-associated protein 1 (80%) and Myb antibody (95%).
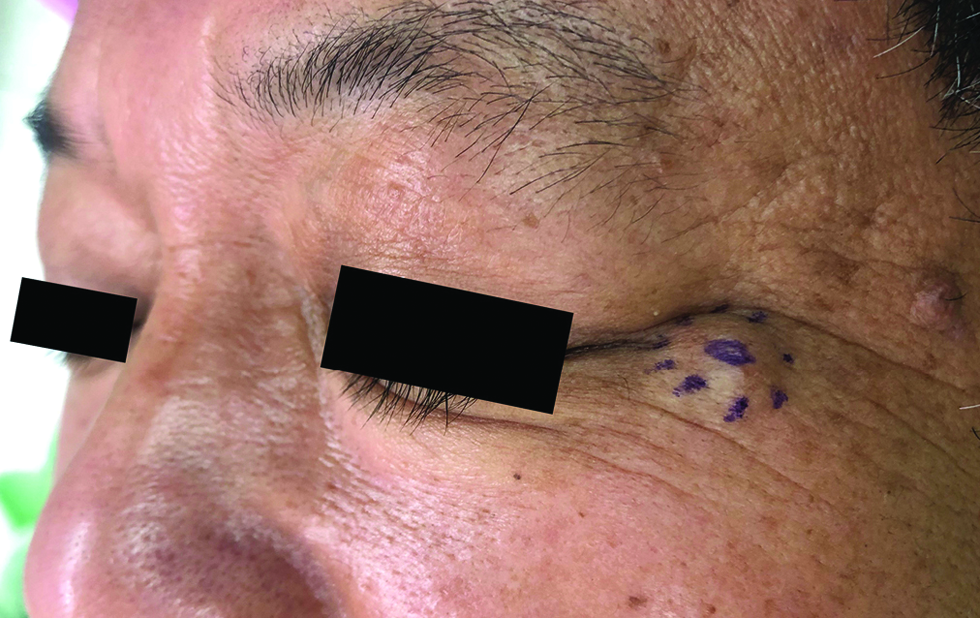
- The smooth, skin-coloured nodule located at the left lateral canthal area in the third case
The majority of endocrine mucin-producing sweat gland carcinoma cases appear to be solitary lesions located at peri-orbital areas. In the study of 63 endocrine mucin-producing sweat gland carcinoma cases by Agni et al.,1 only two cases had tumours on both upper and lower eyelids, and one case had the lesion at the temple area. Indeed, endocrine mucin-producing sweat gland carcinoma has a predilection for the eyelid region, yet no definitive explanation of such localization has been proposed in the literature. For dermatologists and pathologists not familiar with endocrine mucin-producing sweat gland carcinoma, misclassification of such tumours as other entities or even benign lesions may occur. For example, the major diagnostic differentials are hidradenoma and apocrine adenoma for our second case and basal cell carcinoma for the first case. Hidradenoma also has cystic and solid arrangements, but a greater diversity of cell types (such as eosinophilic polygonal cells, clear cells and squamoid cells) is often discernible in a single lesion, with the absence of a neuroendocrine feature. Compared with endocrine mucin-producing sweat gland carcinoma, tumour cells of apocrine adenoma exhibit more prominent nucleoli and more eosinophilic, granular cytoplasm, without mucin production of estrogen receptor/progesterone receptor expression. Basal cell carcinoma frequently shows attachment to the epidermis, with conspicuous palisading of peripheral cells and retraction clefts from the adjacent stroma. All of these entities rarely stain positive for estrogen receptor/progesterone receptor and neuroendocrine markers.
The demonstration of neuroendocrine immunophenotype is sometimes challenging, and the new antibody, insulinoma-associated protein 1, appears superior (at least in sensitivity) to the conventional neuroendocrine markers such as synaptophysin and chromogranin, CD56 and neuron-specific enolase (the latter two also lack specificity). Distinct nuclear expression of Myb antibody is evident in our three cases, echoing with the study done by Held et al., which described 10 cases of endocrine mucin-producing sweat gland carcinoma displaying a strong and homogeneous nuclear expression of Myb antibody.2 The specific genetic event driving Myb antibody overexpression is yet to be unravelled, however, this antibody may serve as a diagnostic adjunct. Another intriguing finding is the positive staining of androgen receptor in these three cases, a phenomenon seldom mentioned in the literature. In breast cancers, there is a higher prevalence of androgen receptor expression among estrogen receptor-positive tumours; and for estrogen receptor-negative/triple-negative subgroups, androgen receptor is more commonly expressed in those showing apocrine differentiation,3 a feature observed in our second case of endocrine mucin-producing sweat gland carcinoma. Regarded as a cutaneous counterpart of breast solid papillary carcinoma, whether the co-expression of estrogen receptor and androgen receptor is universal in endocrine mucin-producing sweat gland carcinoma or restricted to men alone remains to be elucidated on more tumour samples.
Contrary to the female-prone (2:1) incidence reported in the Western world,1 there appears to be a male preference in East Asia. As far as Japan and Taiwan are concerned, only two of 20 cases (including our three patients) are females,4,5 indicating a 9:1, male-predominant ratio. Indeed, more cases are needed to determine if there is truly a gender discrepancy among different racial populations. Similar to the reported excellent prognosis,1 no evidence of tumour recurrence has been found in our patients nine months, 21 months and seven months after tumour excision respectively. Nevertheless, long-term follow-up is still mandatory for its rarity and the undiscovered pathogenesis including genetic alterations.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- An update on endocrine mucin-producing sweat gland carcinoma: Clinicopathologic study of 63 cases and comparative analysis. Am J Surg Pathol. 2020;44:1005-16.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Endocrine mucin-producing sweat gland carcinoma: Clinicopathologic, immunohistochemical, and molecular analysis of 11 cases with emphasis on MYB immunoexpression. J Cutan Pathol. 2018;45:674-80.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic and predictive value of androgen receptor expression in postmenopausal women with estrogen receptor-positive breast cancer: results from the Breast International Group Trial 1-98. Breast Cancer Res. 2019;21:30.
- [CrossRef] [Google Scholar]
- Four male cases of endocrine mucin-producing sweat gland carcinoma: Specific gender differences in East Asia. Kaohsiung J Med Sci. 2020;36:467-68.
- [CrossRef] [PubMed] [Google Scholar]
- Endocrine mucin-producing sweat gland carcinoma: Report of two cases. Dermatologica Sin. 2018;36:262-63.
- [Google Scholar]





