Translate this page into:
Eosinophilic fasciitis induced by natalizumab in a patient affected by multiple sclerosis
Corresponding author: Dr. Vincenzo Maione, Department of Dermatology, Spedali Civili, University of Brescia, Brescia, Italy. maionevincenzo@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Maione V, Miccio L, Sala R, Zambelli C, Calzavara-Pinton P. Eosinophilic fasciitis induced by natalizumab in a patient affected by multiple sclerosis. Indian J Dermatol Venereol Leprol 2021;87:146-146.
Sir,
Eosinophilic fasciitis, or Shulman disease, is an uncommon sclerotic disease of muscular fasciae, with unknown etiology and poorly understood pathogenesis. This disorder has been related to different conditions such as hematologic disorders, solid neoplasms, autoimmune diseases, and infections.1 Different drugs have been reported in recent literature to be related to the development of Shulman disease [Table 1].1 We report a case of a possible correlation between natalizumab and eosinophilic fasciitis.
| Simvastatin |
| Atorvastatin |
| Ramipril |
| Phenytoin |
| Subcutaneous heparin |
| Flu vaccination |
A 51-year-old man presented with a 3-month history of progressive non-pitting edema of the legs. The skin appeared thickened and indurated. The patient also complained of limited joint mobility and myalgia. He had a history of ankylosing spondylitis which was well-controlled with low- dose steroid (5 mg/day of prednisone)––and was also suffering from relapsing-remitting form of multiple sclerosis, resistant to different therapies (high doses of steroids and glatiramer acetate). To achieve a better control of the neurological disease, intravenous therapy with natalizumab (300 mg every 4 weeks) had been started three months earlier. After each infusion, the patient reported the development of painful non-pitting edema, which spontaneously regressed in six/ seven days, without any treatment. After the last infusion, the edema became persistent and hence the patient was hospitalized.
Cardiac, hepatic and renal workup were normal. Both legs and feet showed bilateral sclerosis, with a peau d’orange appearance. On the pretibial region, a superficial indentation along the course of a superficial vein, was seen, compatible with the “groove sign” [Figure 1].
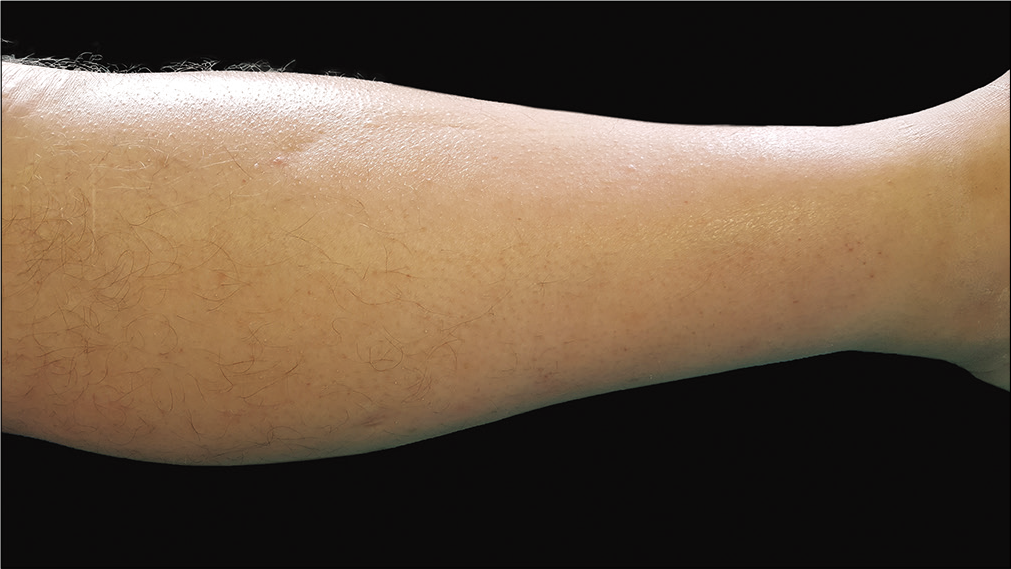
- In left pretibial area presence of slight indentation along the course of superficial vein compatible with “groove” sign
The differential diagnoses included systemic sclerosis, nephrogenic systemic fibrosis, morphea profunda, and scleromyxedema. There was no evidence of Raynaud phenomenon, sclerodactyly, telangiectasia, calcinosis cutis, or gastroesophageal reflux. Laboratory tests showed a high white blood cell count (10940/μL) with mild eosinophilia (900 cells/ μL, 8.6%). C-reactive protein, erythrocyte sedimentation rate, and blood sugar levels were within normal range. Hepatic and muscular enzymes, and thyroid hormones showed no abnormalities. Antinuclear antibodies and extractable nuclear antigen antibodies done to rule out autoimmune connective tisue diseases were negative. Borrelia serology was negative. Acute deep vein thrombosis was ruled out through ultrasound imaging. Magnetic resonance imaging could not be done due to the presence of a metal foreign body in one leg. Cutaneous ultrasound imaging displayed increased signals in the fascia and dermal edema [Figure 2]. Abdominal ultrasound and chest x-ray for neoplasms were negative, ruling out a paraneoplastic etiology.
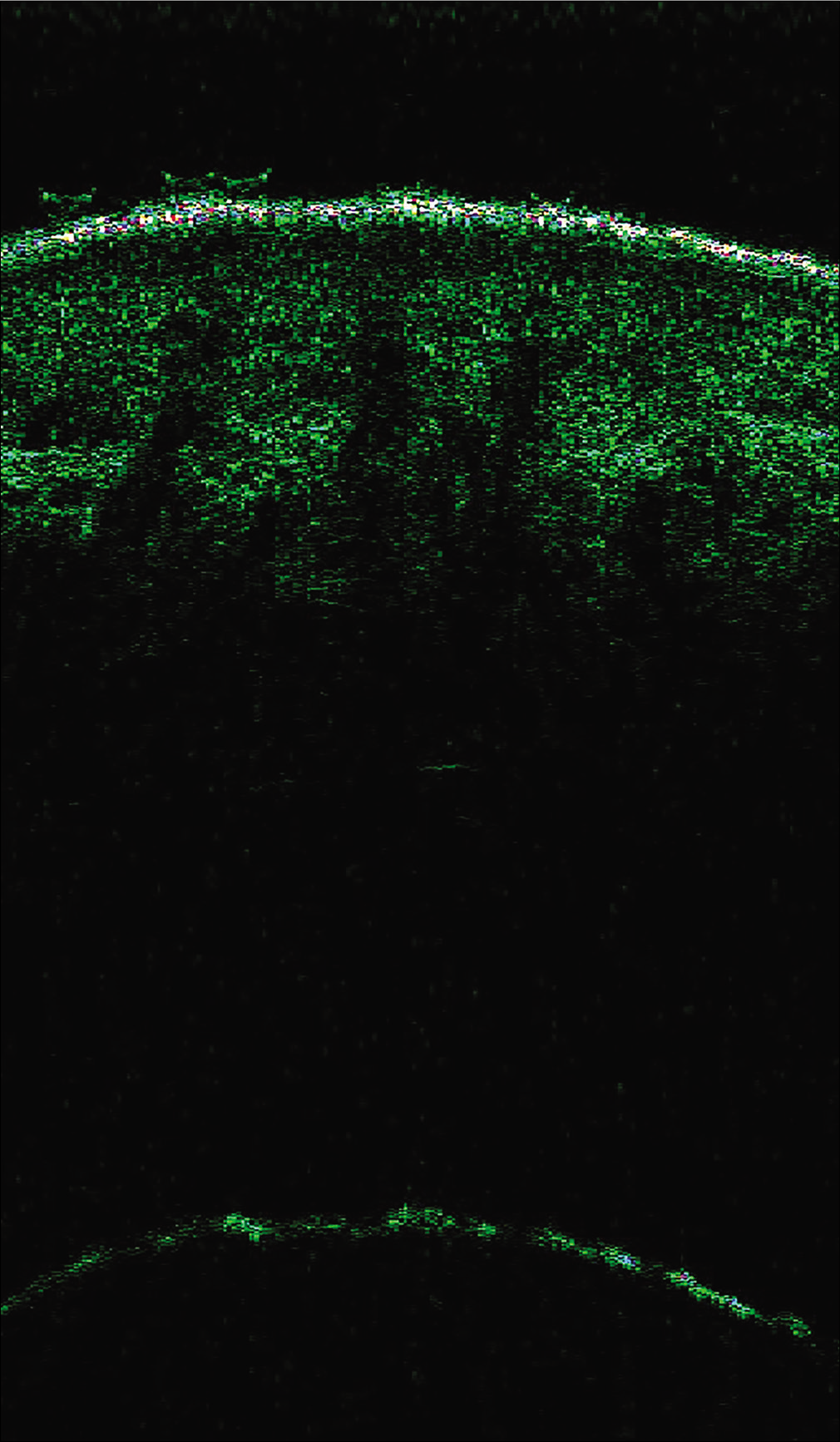
- High-frequency ultrasonography (50 MHz) of affected skin showed dermal edema and increased fascial signal (Skin Scanner, Taberna Pro Medcum, Im Dorf, Leuneburg; usable depth of signal penetration was 8 mm and the gain 40 dB)
Histopathological examination showed orthokeratosis and dermal edema, with a perivascular inflammatory infiltrate and rarely eosinophils [Figure 3a]. More importantly, hypodermal fibrous thickening of septa and eosinophilic infiltrates of fascia were present [Figures 3b and c]. The clinical and histopathological features suggested the diagnosis of drug- related eosinophilic fasciitis, according to the Naranjo Adverse Drug Reaction Probability Scale [Table 2]. After discussing the case with the neurologists, treatment with natalizumab was stopped. The patient was treated with intravenous methylprednisolone (0.6 mg/kg tapered over a period of 6-weeks), along with a three months cycle of UVA 1-phototherapy and physiotherapy, showing rapid improvement of the sclerotic lesions.
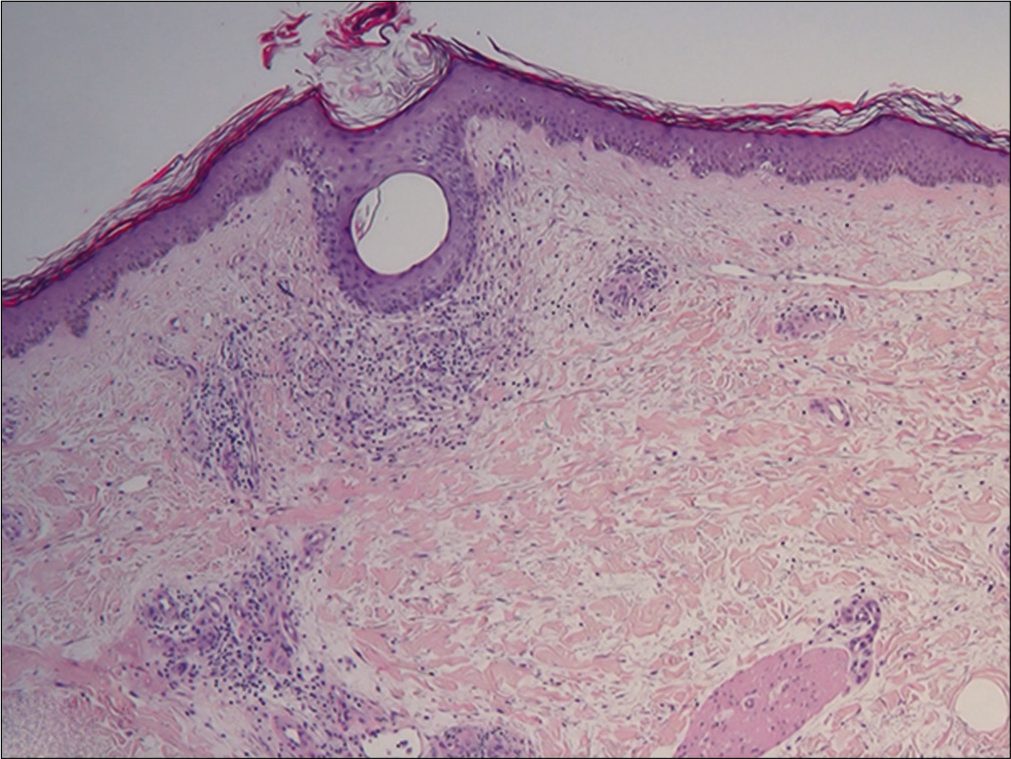
- Presence of hyperkeratosis with orthokeratosis associated with dermal edema and some perivascular inflammatory infiltrates with rare eosinophils (H and E, ×4)
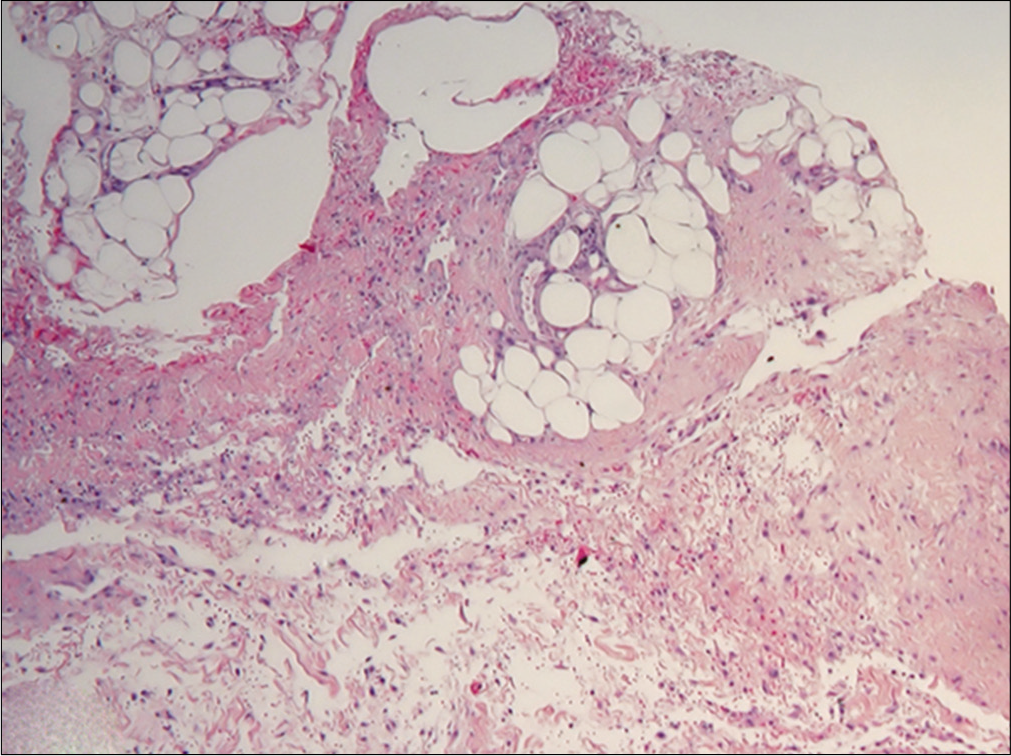
- Sclerotic thickening of septa of subcutaneous fat with presence of lymphocytes and eosinophils in fascia (H and E, ×4)
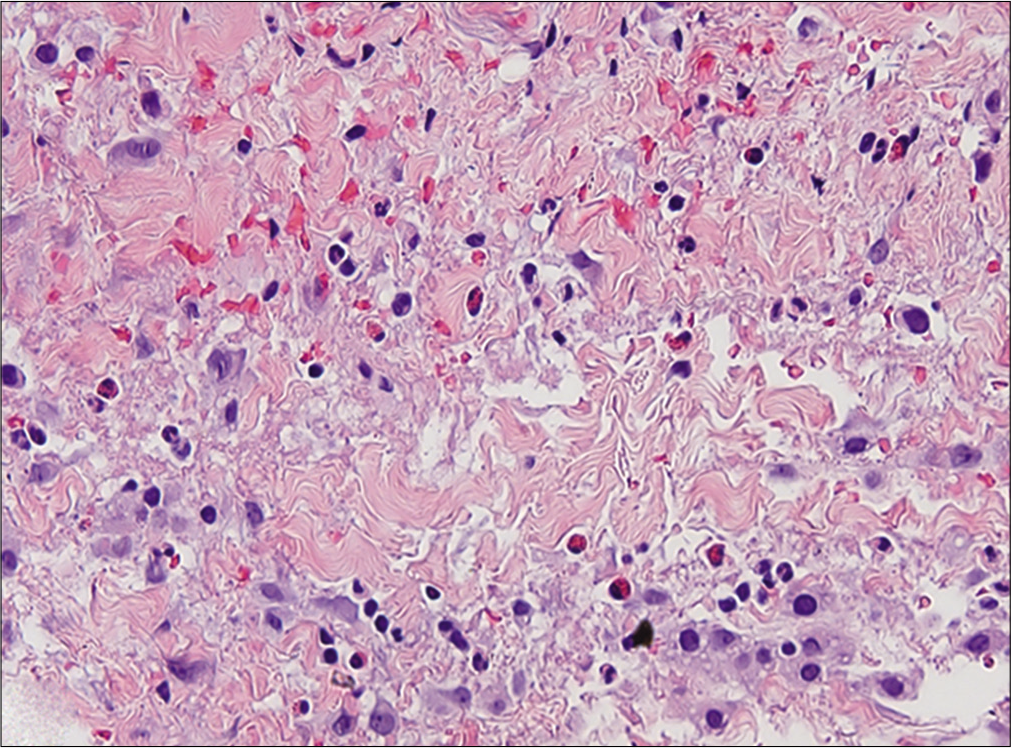
- Infiltrating eosinophils in fascia (H and E, ×20)
| Questions | Yes | No | Do not know | Our case |
|---|---|---|---|---|
| Are there previous conclusion reports on this reaction? | +1 | 0 | 0 | 0 |
| Did the adverse event appear after the suspect drug was administered? | +2 | −1 | 0 | +2 |
| Did the AR improve when the drug was discontinued or a specific antagonist was administered? | +1 | 0 | 0 | +1 |
| Did the AR reappear when drug was re-administered? | +2 | −1 | 0 | +2 |
| Are there alternate causes(other than the drug) that could solely have caused the reaction? | −1 | +2 | 0 | +2 |
| Did the reaction reappear when a placebo was given? | −1 | +1 | 0 | 0 |
| Was the drug detected in the blood(or other fluids) in a concentration known to be toxic? | +1 | 0 | 0 | 0 |
| Was the reaction more severe when the dose was increased or less severe when the dose was decreased? | +1 | 0 | 0 | 0 |
| Did the patient have a similar reaction to the same or similar drugs in any previous exposure? | +1 | 0 | 0 | 0 |
| Was the adverse event confirmed by objective evidence? | +1 | 0 | 0 | +1 |
| Total score | 8 |
Scoring ≥9: Definite, 5-8: Probable, 4-1: Possible, 0: Doubtful. AR: Adverse reaction
Natalizumab is a recombinant humanized monoclonal antibody that acts by binding to the α4 subunit of α4β1 (VLA-4) and α4β7 (LPAM-1) integrins and prevents the adhesion of leukocytes to endothelial cells and their migration through the blood–brain barrier. Although natalizumab may induce mild peripheral blood eosinophilia, eosinophilic peripheral diseases during this treatment are rarely encountered in literature.2 Curto et al. reported the relationship between eosinophilic pneumonia and natalizumab in an asthmatic patient.3 Moreover, Bujold et al. described a case of eosinophilic fasciitis during natalizumab therapy, occuring after intense physical activity. In the latter report Shulman disease did not relapse after subsequent infusion of natalizumab.4 In our case the onset of fasciitis after the drug administration and the rapid recurrence after a rechallenge may confirm the putative role of natalizumab in causing eosinophilic fasciitis.
Although the exact mechanism by which natalizumab causes eosinophilic fasciitis is not known, it is possible that this drug activates Th2 lymphocytes via antigen-dependent/-independent mechanisms or favors the differentiation of hematopoietic progenitors into eosinophils. Consequently, in genetically predisposed individuals or in the presence of trigger factors, these cells increase their capacity to migrate to specific tissues through other adhesion molecules.5 In conclusion, we report a probable case of Shulman disease induced by natalizumab. We suggest to add natalizumab to the list of inductors of eosinophilic fasciitis, though further studies are necessary to confirm this assumption.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Eosinophilic skin diseases: A comprehensive review. Clin Rev Allergy Immunol. 2016;50:189-213.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899-910.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary eosinophilia associated to treatment with natalizumab. Ann Thorac Med. 2016;11:224-6.
- [CrossRef] [PubMed] [Google Scholar]
- Eosinophilic fasciitis occurring under treatment with natalizumab for multiple sclerosis. J Cutan Med Surg. 2014;18:69-71.
- [CrossRef] [PubMed] [Google Scholar]
- Hypereosinophilia in patients with multiple sclerosis treated with natalizumab. Neurology. 2011;77:1561-4.
- [CrossRef] [PubMed] [Google Scholar]





