Translate this page into:
Erythrodermic atopic dermatitis responding to tralokinumab after dupilumab failure
Corresponding author: Aida Lara-Moya, Department of Dermatology, Hospital Parc Taulí de Sabadell, Sabadell, Spain. aidalara3@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Lara-Moya A, Exposito-Serrano V, Garbayo-Salmons P, Sabat Santandreu M. Erythrodermic atopic dermatitis responding to tralokinumab after dupilumab failure. Indian J Dermatol Venereol Leprol. 2025;91:S87-S89. doi: 10.25259/IJDVL_216_2024
Dear Editor,
Interleukins (IL)-4 and IL-13 are cytokines that play a key role in the immunopathogenesis of atopic dermatitis (AD). Dupilumab is a monoclonal antibody targeting the alpha subunit of the IL-4 receptor, effectively blocking signalling from both IL-4 and IL-13. However, tralokinumab is a fully human immunoglobulin G4 (IgG4) monoclonal antibody neutralising IL-13. A common adverse effect of dupilumab is conjunctivitis, whereas this adverse effect is less common with tralokinumab.1 For this reason, in some AD patients with severe head and neck involvement, it is preferable to use tralokinumab over dupilumab. Tralokinumab might be able to treat some of the most severe cases of AD when dupilumab has proven ineffective.1
A 45-year-old man with a history of of BMI of 41, an active smoking habit of 20 cigarettes per day, and asthma under treatment with omalizumab 450 mg every four weeks, was referred to our department due to long-standing AD since childhood, which had significantly worsened over the past 12 months. On examination, the patient exhibited intense and diffuse erythema and scaling, affecting more than 90% of the body surface area (BSA), in addition to severe head and neck involvement [Figures 1a and 1b]. The serum IgE levels were 255 UI/mL. A spongiotic pattern seen in histopathology was supportive of the diagnosis of erythrodermic AD. The patient received treatments involving both topical and oral steroids and methotrexate at a dose up to 17.5 mg weekly. The patient showed only minimal improvement, leading to the prescription of cyclosporine at 3.5 mg/kg daily. However, cyclosporine was discontinued after eight months due to inadequate response. Subsequently, the patient commenced treatment with dupilumab, which initially produced positive results, except for the head and neck area, which remained significantly affected throughout the treatment course. After 12 months, the patient experienced a relapse, causing dupilumab (12 months) discontinuation. At this juncture, tralokinumab (6 months) was initiated, achieving a remarkable improvement in erythema and pruritus, including head and neck involvement and a reduction in BSA to 2 and eczema area and severity index (EASI) to 1. As of a six-month follow-up, the response has been sustained [Figures 1c and 1d].
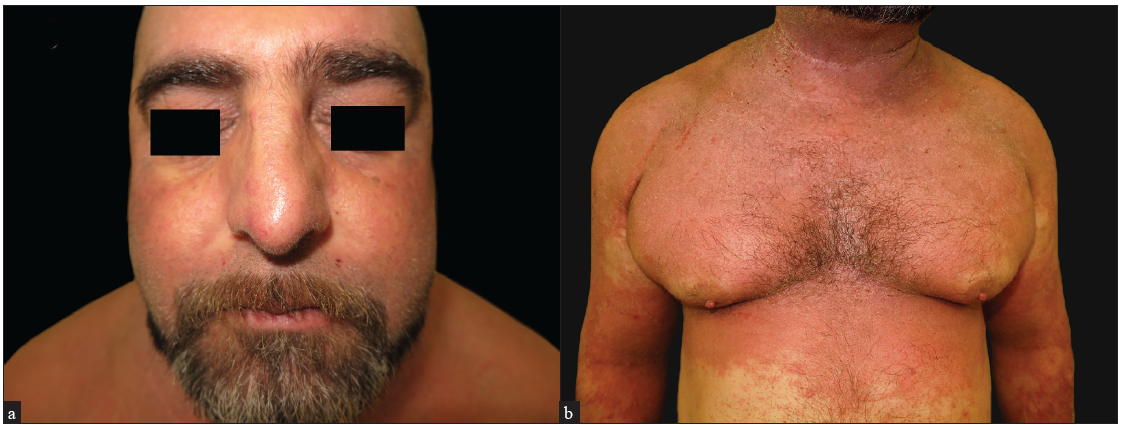
- Intense erythema and scaling on the (a) head and neck region and (b) trunk and upper limbs.
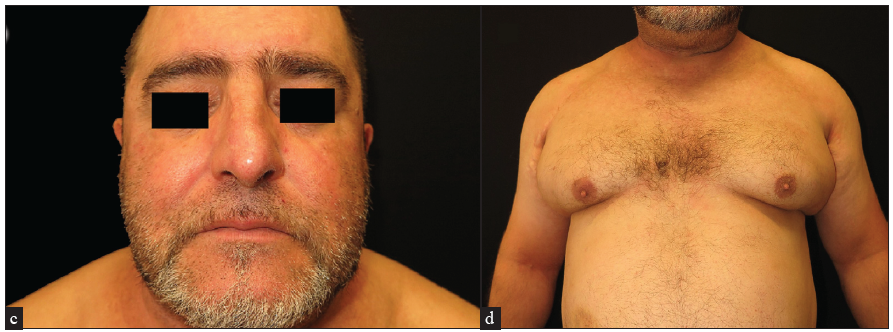
- Clinical improvement seen after 24 weeks of tralokinumab treatment.
The pathogenesis of AD is driven by a cutaneous barrier dysfunction and immune dysregulation involving T helper (Th)2/Th22 lymphocytes and cytokines such as IL-4, IL-5, IL-13, IL-22 and IL-31. However, the AD phenotype varies across ethnicities. Patients of Asian and European American descent show greater involvement of Th1/Th17 activity compared to African American patients who are characterised by Th2/Th22 dominance and significant IgE expression.1,2 In the context of clinical phenotypes, several classifications have been proposed in the medical literature, including classical, nummular dermatitis, prurigo nodular-like, erythrodermic, and psoriasiform, among others.1 Nevertheless, consensus on the preferred nomenclature for these phenotypes remains elusive.
The erythrodermic variant of AD, defined by the presence of erythema covering more than 90% of the BSA, typically spares the palms and soles. This clinical phenotype is associated with a poor quality of life, high rates of hospitalisation and skin infections.1 Clinical trials evaluating the efficacy of tralokinumab have primarily focused on patients suffering from moderate to severe AD without detailing the prevalence of clinical phenotypes.3,4 While the real-world evidence has shown diverse responses to tralokinumab, the erythrodermic variant has not been reported.5,6
There are several hypotheses to explain why dupilumab may fail while tralokinumab could be effective. One possible explanation is that tralokinumab specifically inhibits an epitope of IL-13, preventing its binding to the IL-13Rα1/IL-4Rα receptor and IL-13α2 receptor. In contrast, dupilumab inhibits IL-4 signalling through the type I receptor (IL-4Rα/γc) as well as IL-4 and IL-13 signalling through the type II receptor (IL-4Rα/IL-13Rα). It is possible that in our patient, the role of IL-13 is significant, thus allowing tralokinumab to inhibit IL-13 more effectively than dupilumab. Another explanation could be the presence of anti-drug antibodies against dupilumab, although it is unclear whether they are neutralising and could contribute to secondary treatment failure.7
The limitations of this case are its single-case nature and the inability to conduct a more in-depth analysis of tissue or blood samples to examine the cytokines involved in our patient’s AD.
We present a case of erythrodermic AD successfully treated with tralokinumab. While our case underscores the efficacy of tralokinumab in managing erythrodermic AD, in a singular case who failed to respond to dupilumab, the reasons for this superiority is still unclear. Also, with the regulatory approvals for Janus kinase (JAK) inhibitors, they could be potentially given in such cases of erythrodermic AD, provided there are no contraindications.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
V. Exposito-Serrano has been an investigator, speaker, and/or advisor for AbbVie, Lilly, LEO Pharma, Novartis, and Sanofi Genzyme, and received support for attending meetings and/or travel from AbbVie, Lilly, LEO Pharma, Novartis, and Sanofi Genzyme-P. Garbayo-Salmons declares honoraries for participating in the advisory boards from Novartis; received support for attending meetings and/or travel from AbbVie, Amgen, Lilly, LEO Pharma, Novartis, and UCB.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Nomenclature and clinical phenotypes of atopic dermatitis. Ther Adv Chronic Dis. 2021;12:20406223211002979.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2) Br J Dermatol. 2021;184:437-49.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: Results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184:450-63.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Real-life experience of tralokinumab for the treatment of adult patients with severe atopic dermatitis: A multicentric prospective study. Clin Drug Investig. 2023;43:299-306.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Survival, efficacy and safety of tralokinumab after 32 and 52 weeks of treatment for moderate-to-severe atopic dermatitis in adults: A multicentre real-world study. J Eur Acad Dermatol Venereol. 2024;38:e11-3.
- [CrossRef] [PubMed] [Google Scholar]
- Interleukin-13: Targeting an underestimated cytokine in atopic dermatitis. Allergy. 2020;75:54-62.
- [CrossRef] [PubMed] [Google Scholar]






