Translate this page into:
Evaluation of hypothalamic-pituitary-adrenal axis in patients with atopic dermatitis
2 Department of Endocrinology, Post Graduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
A J Kanwar
Department of Dermatology, Venerology and Leprology, Post Graduate Institute of Medical Education and Research, Sector 12, Chandigarh-160 012
India
| How to cite this article: N, Kanwar A J, Bhansali A, Parsad D. Evaluation of hypothalamic-pituitary-adrenal axis in patients with atopic dermatitis. Indian J Dermatol Venereol Leprol 2011;77:288-293 |
Abstract
Background: Most of the research on atopic dermatitis (AD) has focused on the pathophysiological role of the immune system in AD, and the role of endocrine signals in the pathology of AD has not been explored. Current research has shown a link between the neuroendocrine and immune functions. Aim: The aim was to measure the serum basal cortisol levels and cortisol levels following a low-dose ACTH stimulation test in patients with AD before and after treatment with corticosteroids. Methods: Three groups of patients with AD were evaluated: mild, moderate, and severe. Basal cortisol levels following an ACTH stimulation test were measured before and after treatment with topical steroids when an improvement in the disease activity by 75% as determined by the SCORAD index was observed. Results: Eighteen patients of the severe group at baseline showed an impaired hypothalamic-pituitary-adrenal (HPA) axis with cortisol levels <250 nmol/l during their first visit. A total of 13 of 18 patients regained their HPA axis activity when the baseline cortisol was measured after using topical corticosteroids which resulted in 75% improvement in the disease activity. Conclusions: The disease activity rather than the use of topical costicosteroids is responsible for the low basal levels in patients with severe AD.Introduction
Atopic dermatitis (AD) is an inflammatory skin disease characterized by the hyperactivity of the humoral immune system with an onset in infancy or early childhood. Its prevalence is currently estimated to be in the range of 10-15% in most parts of the world with an increasing incidence in the recent times. [1],[2]
The pathogenesis of AD is multifactorial. A number of predisposing, immunopathogenic, and provoking factors are involved. Predisposing factors include genetics and the disturbance of skin function. Immunopathogenic factors include T-cell dysfunction, biphasic cytokine expression, and the role of immunoglobulin E. Provoking factors include microbial factors, psychosomatic interactions, contact allergens and irritants, inhalant allergens, food, and climate. [3]
Patients with allergic disorders have been noted to have variations in cortisol patterns under natural conditions as well as a differential cortisol response to stress. A growing number of animal data strongly suggest that a hyporeactive hypothalamic-pituitary-adrenal (HPA) axis may be pathologically significant by increasing the susceptibility to chronic inflammation. [4]
HPA axis suppression has been observed after the use of corticosteroids, topical as well as systemic in some studies. [5] This potential adverse effect is a source of concern for the dermatologists since currently topical corticosteroids form the mainstay of therapy for AD. However, in a recent study it has been observed that HPA axis suppression as assessed by basal cortisol levels in patients with AD is related to the disease activity. As the disease improves after intensive therapy with topical corticosteroids, HPA axis functioning is restored. [6]
Stress increasingly has been recognized as an important factor in the pathogenesis of AD, but responses to stress are variable and dependent on the existing psychologic foundation of the patient and family. Stressful events often have been observed to occur before an AD exacerbation. [7],[8] Children with AD have been shown to be more susceptible to stress-induced skin eruptions because of a hyporesponsive HPA axis, which blunts body′s natural ability to produce cortisol and suppress inflammation in response to stress. [9]
The aim of the current study was to investigate basal serum cortisol levels in AD patients with mild, moderate, and severe disease during disease exacerbation and after treatment with topical steroids. Also, the level of HPA axis aberration if any was accessed by performing ACTH stimulation in these patients.
Methods
Sixty-two patients of AD in equal proportion of mild, moderate, and severe cases attending the Pediatric Dermatology Clinic of the Department of Dermatology, Venerology and Leprology at the Nehru Hospital attached to Post Graduate Institute of Chandigarh were enrolled for the study. Patients in the age group of 6 months to 16 years with diagnosis of AD satisfying the criteria by Hanifin and Rajka [10] and who had not used corticosteroids in the past 3 months in any form were included.
Exclusion criteria were the presence of concomitant serious skin disorders, clinically overt skin infection, bronchial asthma, immunocompromised states, and systemic infections.
Investigations
Basal serum cortisol levels in all the patients were determined at 08:00 am on week 0 and repeated when the SCORAD index improved by 75% of the initial score. ACTH, 1 μg I.V., was injected after cannulation. Blood cortisol was measured at 30 and 60 min after injection. This was carried out at week 0 and after an improvement in the SCORAD index of 75% in all the three groups.
A value of 250 nmol/l at 08:00 am was assigned as the cut-off for the basal serum cortisol levels, i.e., 0-min cortisol levels. After 1-μg injection of ACTH, the 30-min cut-off level for serum cortisol was assigned as 550 nmol/l, and for 60 min after the injection, the cut-off level was again assigned as 550 nmol/l. [11],[12]
Disease activity
The severity of patients was recorded according to the SCORAD index as mild, moderate, or severe at week 0 that is, at their first visit. Patients were assessed initially after 2 weeks and later every week with the SCORAD index for their disease activity until their SCORAD index improved by 75% of the initial score. They were followed for a maximum period of 12 weeks. If the patients did not respond to betamethasone within this duration they were excluded from the study.
Statistical evaluation
Statistical analysis was performed using SPSS for Windows 97. The cortisol levels and results of the ACTH stimulation test were correlated with the severity of the disease. The correlation between the cortisol levels before and after treatment was performed. An association between variables was tested using Fisher′s exact test. Differences in median values between independent variables were tested using the Mann-Whitney test. The Spearman rank correlation value was used to measure corelations between variables.
Results
Sixty-two patients diagnosed with AD attending the Pediatric Dermatology Clinic of Department of Dermatology, Venerology and Leprology were enrolled in the study. No patient was lost to follow-up. The age of the study population ranged from 6 months to 16 years with a mean (±SD) age of 6.95 years (±4.50 years). The study population comprised 23 girls (37%) and 39 (62.9%) boys. The total duration of illness was 5.40 years (±3.97 years) with the exacerbation of the symptoms lasting for 1 month (±0.41 months). A family history of atopy was positive in 22 patients (36.5%) and negative in 40 (64.5%). A history of other allergies was positive in 34 (54.8%) patients and was absent in 28 (45.2%) patients.
Disease activity
The mean (±SD) SCORAD index of the mild group was 11.35 (±2.44) on the first visit which decreased to 6.33 (±2.13) 2 weeks after follow-up, and in the final visit was 2.35 (±0.94). The moderate group patients had a mean (±SD) value of 23.95 (±3.25) on the first visit which following treatment decreased to 13.92 (±3.21) and finally was 5.11 (±1.44). The SCORAD index recorded by the severe group during the first visit was 44.27 (±5.60); the second visit showed a score of 23.07 (±6.37) and the final visit showed a score of 10.33 (±3.71)..
Topical corsticosteroid use
The mild group used a mean of 155 g (±25 g) of topical steroid (0.10% betamethasone valerate) over the period of treatment. The moderate group used 250 g (±25 g) of topical steroid for 75% improvement in the disease activity. The severe group used 450 g (±45 g) of topical steroid during the study period.
Cortisol cut-offs for the mild AD group
In mild AD, there were eight patients showing values below the cut-off on the first visit. After improvement in the disease, 10 patients showed values below the cut-off. This was not found to be statistically significant (P-value 0.727). When the levels of serum cortisol after 30 min of 1-μg ACTH stimulation were measured, the following results were seen. All the values were > 550 nmol/l during the first visit. On follow-up, after the last visit, five values were found to be < 550 nmol/l. This was found to be tending toward statistical significance (P-value 0.063; [Table - 1]).
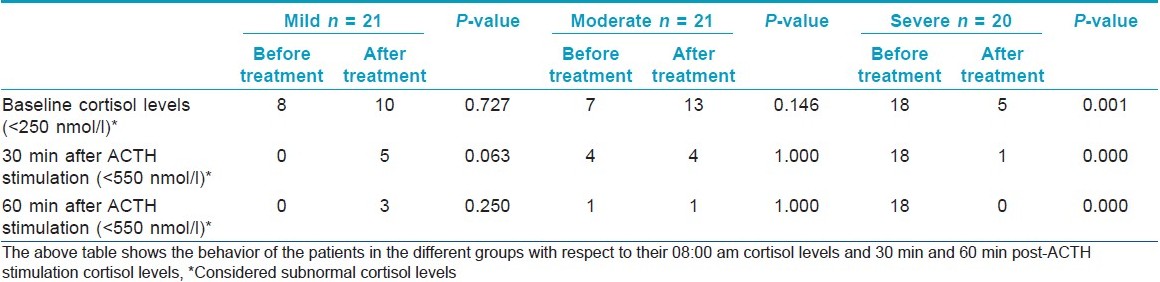
Cortisol cut-offs for the moderate AD group
In the moderate group, the behavior of cortisol levels was found to be slightly different from the mild group. The baseline cortisol level of <250 nmol/l was recorded in seven patients during the first visit. The repeat baseline cortisol after 75% disease improvement was <250 nmol/l in 13 patients. These findings on further analysis were not found to be statistically significant (P-value = 0.146). In four patients 30 min following ACTH stimulation both at the first visit and during the follow-up visit, a level of <550 nmol/l was recorded. The difference was not found to be statistically significant (P-value = 1.000).
Cortisol cut-offs for the severe AD group
The majority, i.e., 18 patients, of the severe group showed cortisol levels of <250 nmol/l at baseline during their first visit. The number of patients decreased to 5 when the baseline cortisol was remeasured after 75% improvement in the disease activity. This was found to be statistically significant (P-value = 0.001). After 30 min of the ACTH stimulation test, 18 patients of the severe group failed to respond, with their cortisol levels being <550 nmol/l. This changed drastically after improvement in their disease status when only 1 out of 20 patients failed to respond. A statistically significant difference was found with a P-value <0.001.
Discussion
In this prospective, parallel cohort study of 62 AD patients, it was demonstrated that half the patients with AD have low basal cortisol levels and this is more marked in patients with severe AD. Patients with severe AD had low basal serum cortisol levels and post ACTH stimulated cortisol levels at baseline even before initiation of treatment with topical steroids. After the application of topical steroids, there was a recovery of the HPA axis in 98% of patients.
The 08:00 am serum cortisol level of <250nmol/l was taken as the cut-off for adequate adrenal gland functioning. [11] In 20 patients with severe AD, the baseline serum cortisol level at 08:00 am was 90.85 nmol/l before treatment; out of those, 18 patients had a morning value of <250 nmol/l and even after 1-μg ACTH stimulation, they did not qualify for the optimal reserve volume (>550 nmol/l). After treatment, the mean serum cortisol levels improved in this group and only five patients had a baseline serum cortisol level of <250 nmol/l and interestingly all patients had a serum cortisol level of >550 nmol/l after 1-μg ACTH stimulation suggesting the recovery of the HPA axis.
The above observations seem to be consistent with the results of the study by Elison [13] et al., in which 35 patients were divided into four groups depending on whether they received mild, moderate, or potent/very potent topical preparations with group 4 receiving steroids through other routes too. They found that on low-dose synacthen stimulation test, the groups receiving mild and moderate topical steroids responded but the other two groups failed to show an adequate response.
Topical betamethasone valerate was not found to cause significant adrenal suppression in any of the three groups. A literature search gives conflicting results with studies showing strong evidence of adrenal hypofunction with clinically reversible adrenal insufficiency observed on rare occasions following a topical steroid application. In a study involving 18 patients of AD with greater than 30% body surface involvement using twice daily preparation in an ointment form, no HPA axis suppression was found after a continuous 4-week application. [14] Risk factors for adrenal suppression have been elucidated in the form of use of high-potency corticosteroids, occlusive or prolonged treatment application, and use in thin-skinned areas. [15]
In a recent study where 25 patients with severe AD who needed hospitalization and 28 outpatients with moderate disease were compared for their basal cortisol levels, it was found that inpatients′ cortisol levels were significantly lower than those of the outpatient group. It was also found that at the time of discharge, there was a significant increase in the basal cortisol level in the inpatient group. [6]
Another study was performed with a total of 45 severe AD patients who were hospitalized and divided into two groups based on the history of receiving a topical steroid in prior 3 months (group 2) and no history of receiving topical steroids (group 1). The serum cortisol levels and response to ACTH was found to be decreased in both the groups and this improved following treatment with topical steroids only in group 1 while no increase or decrease in cortisol levels or response to ACTH was found in group 2. They concluded that the suppression of the adrenal cortex was the result of both percutaneous absorption of corticosteroids as well as other factors related to the disease. [16]
In the immune system, neuropeptides are involved in the integration of intraimmune regulation and in the bidirectional communication between the immune and the neuroendocrine systems. Neuropeptides are ubiquitous throughout the body. When the higher cortical centers are activated by stress, there is an increased secretion of substance P from the adrenal glands. [17] Centrally, it serves as a brain peptide that is easily released by psychosocial stress, triggering or exacerbating itching, especially in patients with AD. [18]
Newborns with atopic predisposition have been observed to have a hyperreactivity of their HPA axis while in long-term AD patients, a hyporeactivity of the same has been documented. [19] This phenomenon can be explained by switching of the HPA axis from a hyperreactive state to a hyporeactive state. While there are no data yet to support this hypothesis directly, there are some factors which can explain this. The long-term release of proinflammatory cytokines decreases HPA responsiveness by increasing the negative feedback secondary to an increased endogenous cortisol level and chronic stress has been linked to a blunted HPA axis in studies of animal models. [19]
In another study, AD patients showed reduced responsiveness of the HPA axis and increased reactivity of the sympathetic adrenomedullary system. It may be assumed that the inability to exert an appropriate HPA axis or SAM system response and to generate an adequate regulatory signal for the immunological target cell could increase the risk for aberrant immune functioning, especially under stressful conditions. Also, recent findings suggest that the activation of both systems appears to promote TH 2 cell function and suppress TH 1 cell activity probably via the suppression of IL-12. [20]
The following conclusions can be drawn from this study. Topical mid-potent corticosteroids do not cause a depression of the HPA axis when used over an average of 3 months to control disease exacerbation in patients with AD. In patients with severe exacerbation, the serum cortisol levels are suppressed by the disease activity per se and not due to the application of topical corticosteroids. There is a brisk recovery in the basal cortisol levels when the patients′ disease activity improved by 75%.
When the ACTH stimulation test was performed it was found that patients with severe AD did not show a significant response when the disease was active. This could indicate that during disease exacerbation, the adrenals are not adequately responding to stimulation. Therefore, patients with severe AD may need glucocorticoid replacement during periods of stress. Paradoxically, when the ACTH stimulation test was repeated after recovery from the disease exacerbation in the severe group response was recorded in all the patients. This indicates that HPA axis recovered following an improvement in the disease severity.
In the mild and moderate group even though following topical steroid treatment there was a marginal increase in the number of patients whose cortisol levels decreased below the threshold levels for the basal cortisol activity, this was not found to be statistically significant. Post-ACTH stimulation test values at 30 min were concordant with the 60-min response and therefore 30-min values are validated to see the response following ACTH stimulation.
The basal cut-off was 250 nm/l taken at 08:00 am and all those patients who had a value of ≥ 250 nmol/l qualified the ACTH stimulation test also as a value of ≥550 nmol/l was defined. However, out of 33 patients who had basal cortisol levels <250 nmol/l, only 11 qualified and the rest did not suggesting that the 08:00 am value of ≥ 250nmol/l has 100% PPV which is in consonance with the literature showing a sensitivity of 95% in various studies.
Conclusions
Corticosteroids in various forms are the mainstay of treatment for patients with AD. There remains a phobia among public and doctors about the use of steroids for the various side effects that they may cause in the patients especially adrenal suppression. This study makes a strong case for the use of topical corticosteroids in patients with severe AD without the complication of HPA suppression by the steroids themselves. In fact, the steroids act as a supplement to the body when the HPA axis is hyporeactive during active inflammation as seen in this study. Not all patients in AD may develop severe disease during their disease course but the ones who do develop severe AD may represent a subset of population which is inherently susceptible to severe disease because of the deregulatory HPA axis. In this particular subset, it may actually be worthwhile to keep the patients on regular topical steroids to prevent the risk of acute exacerbations without any HPA axis suppression issues. Therefore, the practical utility of this study lies in the fact that when doctors describe strong topical corticosteroids in patients with severe AD, they may do so without the fear of HPA axis suppression. Also, this may be the reason why topical calcinuerin inhibitors are found to be not so effective in controlling severe AD.
More trials will be needed to further confirm these results. This study brings out an interesting concept in the pathophysiology of severe AD which needs further studies to document if the HPA axis attenuation is one of the causes for chronic inflammation in AD or is the result of inflammation.
Acknowledgment
The authors like to thank Dr. S. D. Mehta for helping in recruiting patients to this study.
| 1. |
Schultz Larsen F, Diepgen T, Svensson A. The occurrence of atopic dermatitis in North Europe: An international questionnaire study. J Am Acad Dermatol 1996;34:760-4.
[Google Scholar]
|
| 2. |
Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol 2000;43:649-55.
[Google Scholar]
|
| 3. |
Meagher LJ, Wines NY, Cooper AJ. Atopic dermatitis: Review of immunopathogenesis and advances in immunosuppressive therapy. Australas J Dermatol 2002;43:247-54.
[Google Scholar]
|
| 4. |
Chrousos GP. The hypothalamus-pituitary-adrenal axis and immune mediated inflammation. N Engl J Med 1995;332:1351-62.
[Google Scholar]
|
| 5. |
Scoggins RB, Kliman B. Percutaneous absorption of corticosteroids: Systemic effects. N Engl J Med 1965;273:831-40.
[Google Scholar]
|
| 6. |
Haeck IM, Timmer-de Mik L, Lentjes EG, Buskens E, Hijnen DJ, Guikers C, et al. Low basal serum cortisol in patients with severe atopic dermatitis: potent topical corticosteroids wrongfully accused. Br J Dermatol 2007;156:979-85.
[Google Scholar]
|
| 7. |
Brown DG. Stress as a precipitant factor of eczema. J Psychosom Res 1972;16:321-7.
[Google Scholar]
|
| 8. |
King RM, Wilson GV. Use of dairy technique to investigate psychosomatic relations in atopic dermatitis. J Psychosom Res 1991;35:697-706.
[Google Scholar]
|
| 9. |
Buske-Kirschbaum A, Geiben A, Hellhammer D. Psychobiological aspects of atopic dermatitis: An overview. Psychother Psychosom 2001;70:6-16.
[Google Scholar]
|
| 10. |
Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol 1980;92:44-7.
[Google Scholar]
|
| 11. |
Inder WJ, Hunt PJ. Glucocorticoid replacement in pituitary surgery: Guidelines for peri operative assessment and management. J Clin Endocrinol Metab 2002;87:2745-50.
[Google Scholar]
|
| 12. |
Abdu TA, Elhadd TA, Neary R, Clayton RN. Comparison of the Low Dose Short Synacthen Test (1 µg), the Conventional Dose Short Synacthen Test (250 µg), and the Insulin Tolerance Test for Assessment of the Hypothalamo-Pituitary-Adrenal Axis in Patients with Pituitary Disease. J Clin Endocrinol Metab 1999;84:838-43
[Google Scholar]
|
| 13. |
Ellison JA, Patel L, Ray DW, David TJ, Clayton PE. Hypothalamic pituitary-adrenal function and glucocorticoid sensitivity in atopic dermatitis. Pediatrics 2000;105:794-9.
[Google Scholar]
|
| 14. |
Franz TJ, Parsell DA, Halualani RM, Hannigan JF, Kalbach JP, Harkonen WS. Betamethasone valerate foam 0.12%: A novel vehicle with enhanced delivery and efficacy. Int J Dermatol 1999;38:628-32.
[Google Scholar]
|
| 15. |
Levin C, Maibach HI. Topical Cortico-steroid induced adrenocortical insufficiency: Clinical implications. Am J Clin Dermatol 2002;3:141-7.
[Google Scholar]
|
| 16. |
Matsuda K, Katsunuma T, Iikura Y, Kato H, Saito H, Akasawa A. Adrenocortical functions in patients with severe atopic dermatitis. Ann Allergy Asthma Immunol 2000;85:35-9.
[Google Scholar]
|
| 17. |
Tobin D, Nabarro G, Baart de la Faille H, van Vloten WA, van der Putte SC, Schuurman HJ. Increased number of immunoreactive nerve fibers in atopic dermatitis. J Allergy Clin Immunol 1992;90:613-20.
[Google Scholar]
|
| 18. |
Panconesi E, Hautmann G. Psychophysiology of stress in dermatology. The psychobiologic pattern of psychosomatics. Dermatol Clin 1996;14:399-421.
[Google Scholar]
|
| 19. |
Buske-Kirschbaum A. Endocrine and immune responses to stress in chronic inflammatory skin disorder. In: Ader R, editor. Psychoneuroimmunology, 4 th ed. New York: Academic Press; 2007. p. 975-99.
[Google Scholar]
|
| 20. |
Buske-Kirschbaum A, Geiben A, Höllig H, Morschhäuser E, Hellhammer D. Altered responsiveness of the hypothalamus-pituitary-adrenal axis and the sympathetic adrenomedullary system to stress in patients with atopic dermatitis. J Clin Endocrinol Metab 2002;87:4245-51.
[Google Scholar]
|
Fulltext Views
4,068
PDF downloads
3,107





