Translate this page into:
Evaluation of the combination of BP180-NC16a enzyme-linked immunosorbent assay and BP230 enzyme-linked immunosorbent assay in the diagnosis of bullous pemphigoid
2 Department of Dermatology, Shandong Provincial Hospital for Skin Diseases; Shandong Provincial Key Lab for Dermatovenereology, Jinan, China
3 Department of Dermatology, Shandong Provincial Institute of Dermatology and Venereology, Shandong Provincial Academy of Medical Science; Shandong Provincial Hospital for Skin Diseases; Shandong Provincial Key Lab for Dermatovenereology; Shandong Provincial Medical Center for Dermatovenereology, Jinan, Shandong Province, China
Correspondence Address:
Furen Zhang
Shandong Provincial Institute of Dermatology and Venereology, 27397 Jingshi Road, Jinan, Shandong Province, 250022
China
| How to cite this article: Yang B, Wang C, Chen S, Chen X, Lu X, Tian H, Yu M, Zhang D, Shi Z, Zhou G, Zhang F. Evaluation of the combination of BP180-NC16a enzyme-linked immunosorbent assay and BP230 enzyme-linked immunosorbent assay in the diagnosis of bullous pemphigoid. Indian J Dermatol Venereol Leprol 2012;78:722-727 |
Abstract
Background: Bullous pemphigoid (BP) is an acquired autoimmune subepidermal blistering disease characterized by circulating IgG autoantibodies directed against BP180 and BP230 hemidesmosomal proteins. Previous studies have demonstrated that antibodies against the NC16a domain of BP180 mediate BP pathogenesis, while antibodies against BP230 enhance the inflammatory response. Recently, commercial BP180-NC16a enzyme-linked immunosorbent assay (ELISA) and BP230 ELISA kits were developed to detect anti-BP180 and anti-BP230 autoantibodies in human BP sera. Aims: To evaluate the efficacy of BP180-NC16a ELISA and BP230 ELISA in the initial diagnosis of BP. Methods: Sera from 62 BP patients and 62 control subjects were tested by BP180-NC16a ELISA and BP230 ELISA and compared with findings from indirect immunofluorescence (IIF) and immunoblotting (IB) to determine the sensitivity and specificity of these assays. Results: The sensitivities of BP180-NC16a ELISA and BP230 ELISA were 87.1% (54/62) and 56.5% (35/62), respectively, and the specificities of both were 100% (62/62). Using both ELISAs for diagnosis increased the sensitivity to 95.2% (59/62) and was statistically comparable with IB sensitivity. Conclusions: ELISA is a convenient, effective, and reliable method for serodiagnosis of BP, and combined use of BP180-NC16a ELISA and BP230 ELISA can increase the sensitivity of this diagnostic approach.Introduction
Bullous pemphigoid (BP) is a subepidermal autoimmune bullous dermatoses (SABD) characterized by linear deposition of IgG and C3 immune complexes at the cutaneous basement membrane zone (BMZ). [1] Antibodies in BP target the hemidesmosomal components BP180 (also termed BPAG2 and type XVII collagen) and BP230 (also termed BPAG1). [2] The BP180 extracellular domain, NC16a, harbors a stretch of non-collagenous (NC) sequences that also act as the immunodominant site. [3] Rabbit antibodies against mouse NC16a domains have been demonstrated to induce BP-like blisters in neonatal mice via passive transfer. [4] Anti-BP180-NC16A autoantibodies have also been shown to induce BP-like subepidermal blisters in humanized NC16a mice. [5] Collectively, these observations indicate that IgG autoantibodies against BP180 play a critical role in the pathogenesis of human BP. BP230, on the other hand, is a component of the intracellular hemidesmosomal plaque, and exposure to autoantibodies leads to an enhanced inflammatory response of BP. [6]
BP is currently clinically diagnosed by the findings of clinical manifestation, histology, direct immunofluorescence (DIF), and indirect IF (IIF). Some studies have reported that IIF on sodium chloride-split skin (SSS) represents a simple and accurate laboratory method to differentiate BP from the immunohistologically similar but immunologically distinctive epidermolysis bullosa acquisita (EBA); [7] however, this method is not a sensitive indicator of BP clinical activity. [8] Immunoblotting (IB) is an effective method employed to confirm the diagnosis of autoimmune blistering skin diseases by detecting particular autoantigens according to their molecular weights, but the procedure is technically complicated and time-consuming. [9] Enzyme-linked immunosorbent assay (ELISA) has been demonstrated as a useful tool to diagnose BP. The recent commercial release of two ELISA kits using recombinant BP180-NC16a and BP230 proteins as the antigenic substrates appears to provide an easy and rapid approach for detecting the pathogenic anti-BP180 and anti-BP230 antibodies in BP sera. [10],[11]
The present study was designed to assess the efficacy of these commercially-available BP180-NC16a and BP230 ELISA kits for diagnosing BP in Chinese patients. Our results are applicable to the clinical diagnosis of BP as these kits have yet to be definitively tested for their efficacy in a Chinese-specific BP population, and they represent a substantially cost- and time-effective method for BP diagnosis.
Methods
Serum samples
From January 2006 to December 2008, serum samples were collected from 62 patients with BP (mean age: 63.6 years, age range: 5-83 years; male/female: 31/31) and 62 age- and sex-matched control subjects. The controls were composed of 32 healthy individuals with no history of autoimmune disorders, 19 patients with pemphigus vulgaris (PV) and 11 patients with pemphigus foliaceus (PF). Serum was stored at -80°C until use, and all experimental analysis was performed between January 2009 and June 2009.
BP sera were obtained from patients prior to initiation of systemic corticosteroid and immunosuppressive therapy. The median duration of BP at the time of taking blood samples was 3 months (range: 0.2-36.0 months). BP diagnosis was made according to the following criteria: (i) Typical tense cutaneous blisters; (ii) histopathologic examination showing a subepidermal blister; (iii) linear IgG and/or C3 deposition at BMZ by DIF; and (iv) linear IgG and/or C3 deposition at the epidermal side of SSS by IIF or anti-BP180 and/or anti-BP230 antibodies detected by IB. BP diagnosis was made only when all four criteria were fulfilled. The diagnosis of pemphigus was confirmed by the histological finding of an acantholytic intra-epidermal cleft and by DIF-positive staining of the peri-bullous cutaneous biopsy (intercellular deposits of IgG and C3 antibodies). All patients with pemphigus tested positive by IIF for circulating skin antibodies. This study was approved by the Medical Ethics Committee of Shandong Provincial Hospital for Skin Diseases.
Salt-split immunofluorescence
Fresh normal adult skin obtained from patients undergoing the operation was cut into 1.0 cm × 1.0 cm fragments and washed with phosphate-buffered saline (PBS). Then, the fragments were incubated in 1.0 mol/L sodium chloride solution for 48-72 h at 4°C. Cryostat sections (4 μm) were made from the salt-split normal human skin, and IIF was performed as previously described. [12] Patient-matched serum sample was serially diluted at 1:10 and applied to the slides with SSS.
Immunoblotting
The epidermis and dermis of fresh normal adult skin were separated by application of 1.0 mol/L sodium chloride solution. Epidermal extract was prepared as previously described by Mueller et al[13] with the following modifications. The intact epidermis was placed in extraction buffer and mechanically homogenized. The homogenate was subjected to three freeze-thaw cycles (liquid nitrogen and 20°C water bath) and then centrifuged (13000 × g, 30 min, 4°C) to pellet the cell debris. The supernatant was collected and stored at -80°C until use.
Epidermal proteins were separated by 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electro-transferred to a nitrocellulose membrane. The blotted membrane was cut into strips and sequentially incubated with: (i) 5% dried milk in Tris-buffered saline (TBS: 10 mmol/L Tris-HCL, 140 mmol/L NaCl, pH 7.4) for 3 h at room temperature; (ii) 1:10 dilution of each serum in milk/TBS for 3 h at 37°C; and (iii) 1:500 dilution of peroxidase-conjugated rabbit anti-human IgG (Dako, Denmark) for 1 h at 37°C. The strips were washed between each incubation step with a TBS solution containing 0.1% Tween-20. The immunoblots were developed with 3,3-diaminobenzidine (DAB)-4 HCl (0.5 mg/mL) in 10 mmol/L Tris-HCL, pH 7.6 containing 0.03% H 2 O 2 .
Enzyme-linked immunosorbent assay
Concentrations of IgG autoantibodies in the patients′ sera directed against the BP180-NC16a and BP230 were measured using the BP180-NC16a ELISA and BP230 ELISA kits (Medical and Biological Laboratories Co. Ltd., Japan). The BP180-NC16a kit uses the NC16a domain of BP180 produced as fusion proteins in a baculovirus expression vector system. [14],[15] In addition, the BP230 kit used two recombinant proteins as a solid phase, one for the N-terminus and one for the C-terminus of BP230 produced in a bacterial expression system. [15],[16] Following the manufacturer′s instructions, serum samples were diluted at 1:101 and all samples were tested in duplicate. The absorbance (optical density, OD) of each well was read at 450 nm by an automated microplate reader (Bio-Rad 680, Bio-Rad Laboratories, USA). The mean OD of duplicate test sera was calculated for each sample. Results were evaluated as an index value using the following equation: [(OD 450 sample - A 450 negative control)/(A 450 positive control - A 450 negative control)]×100. An index value greater than 9.0 indicated a positive predicted value.
Statistical analysis
A 2 × 2 contingency table Chi-squared test was used to evaluate significant differences between results from IIF, IB, BP180-NC16a ELISA, and BP230 ELISA. P < 0.05 indicated statistical significance.
Results
Among the 62 BP sera evaluated, 52 (83.9%) were positive by IIF-SSS. Among these, 43 (69.4%) stained on the epidermal side, 3 (4.8%) stained on the dermal side, and 6 (9.7%) stained on both sides. Nearly all sera (61/62; 98.4%) had IB-detectable antibodies against BP180 and/or BP230. The sensitivities of BP180-NC16a ELISA and BP230 ELISA for BP samples were 87.1% (54/62) and 56.5% (35/62). When both kits were used in combination, however, the sensitivity rose to 93.5% (58/62). No serum sample from the non-BP control group was positive by IIF-SSS, IB, or ELISA. Thus, the sensitivity of combined BP180-NC16a ELISA and BP230 ELISA was very close to the highly-sensitive IB method and significantly better than that of IIF-SSS.
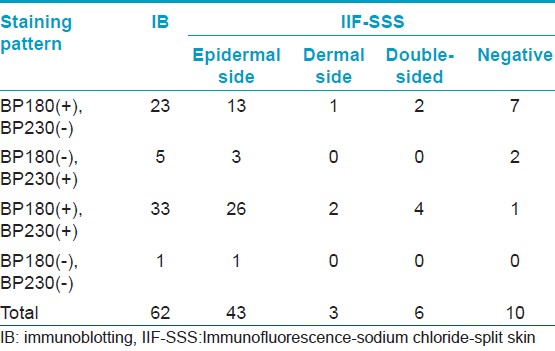
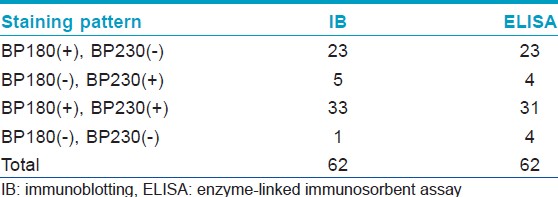
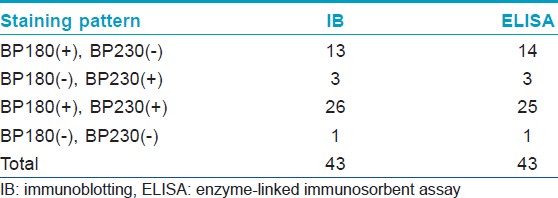
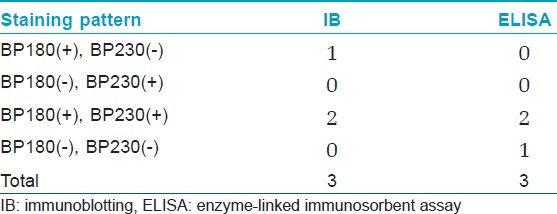
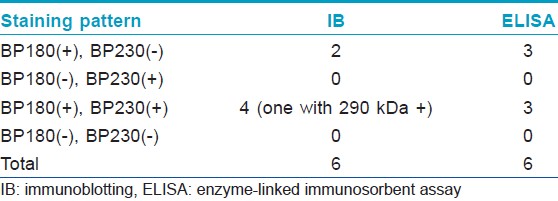
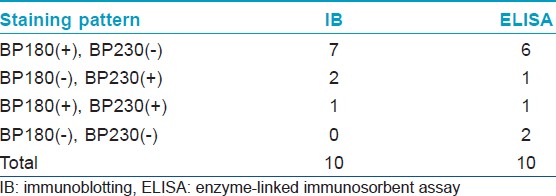
[Table - 1] shows the results from IB and IIF-SSS testing of the 62 BP sera. Since some sera bound only the dermal side in IIF-SSS, these patients might have been misdiagnosed as BP-negative by an IIF-SSS approach using only epidermal samples, a time- and cost-saving measure sometimes employed at resource-limited facilities or when a dermal sample is scarce. [Table - 2] shows the comparison of the results from IB and ELISA testing of the 62 BP sera. [Table - 3], [Table - 4] and [Table - 5] give detailed findings of each, including the staining side (epidermal, dermal, double-sided) of IIF-SSS. The BP180-NC16A ELISA-positive results correlate with the IB-detection of a 180 kDa band, while the BP230 ELISA-positive results correlate with the IB-detection of a 230 kDa band. One of the BP serum samples bound to both the 180 kDa and 230 kDa bands on the epidermal side, but bound to a 290 kDa on the dermal side, indicating that this patient may suffer from BP and EBA simultaneously [Table - 5]. Ten of the BP patients were negative by IIF-SSS, but positive by IB (n = 10) and ELISA (n = 8) [Table - 6], further indicating the particularly low sensitivity of IIF-SSS and comparable sensitivities of IB and ELISA.
Discussion
The commercial BP180-NC16a ELISA kit has previously been evaluated for its ability to diagnose BP in a Chinese patient population. [17],[18] However, in this study, we sought to evaluate for the first time the efficacy of the BP230 ELISA kit and the potential of enhancing BP diagnosis sensitivity by using both kits in combination in Chinese BP patients. Since studies on Chinese-specific BP features are scarce, it is possible that the cytokine profile or related anti-genicities are distinctive from other ethnicities. In fact, two specific polymorphisms in the IL-1 cytokine have been identified as associated with BP development in Chinese women; [19] however, it is unknown whether other Chinese-specific genetic abnormalities exist in the genomes of BP patients. Testing of these two kits in a Chinese-specific population is a necessary first step towards generating a more effective and cost-saving strategy for BP diagnosis in Chinese patients.
The sensitivity of the BP230 ELISA kit was significantly lower than that of the BP180-NC16A ELISA in our patient population. This different sensitivity between the two kits may be a reflection of the following features of our study design or study population. First, all of the patients included in our research were in the initial disease stage. Antibodies to BP180 are known to initiate blister formation in BP; therefore, an increased level of anti-BP180 antibodies would be expected at the initial stage. In contrast, levels of antibodies to BP230 are mostly increased during the remission stage since anti-BP230 antibodies are believed to primarily enhance the inflammatory response. [6],[16] Second, most of our patients were classified as having very severe disease, according to the presence of lesions involving at least 30% of the total skin area. It is well-documented that the level of anti-BP180-NC16a IgG directly correlates with disease activity. [17],[20],[21] Thus, the titer of anti-BP180-NC16A IgG detected by ELISA is expected to be higher among the patients with higher disease activity. A previous study in a Japanese cohort reported that carrying out BP diagnosis with both the BP180-NC16A ELISA and BP230 ELISA kits increased the sensitivity to 95.2%. [16] We also found that the combined ELISA kit procedures increased the sensitivity to above 90%.In addition, the BP180-NC16A ELISA in this study was less sensitive than another Chinese cohort, [18] possibly reflecting the fact that the previous cohort was composed solely of sera samples positive by IIF-SSS.
In other previously published studies using non-Chinese populations, the results of BP180 and BP230 ELISAs in BP were heterogeneous. Zillikens et al[22] showed that the NC16a domain harbors multiple antigenic sites and reacts with 94% of BP sera by ELISA. Hence, an ELISA utilizing an eukaryotic form of the extracellular domain of BP180, as opposed to a prokaryotic form, was more sensitive and specific in the detection of BP180-specific antibodies from human patients. [23] Both studies used the same method as our current study but with different kits. Bernard et al[24] demonstrated that a high-titer anti-BP180 ELISA score and, to a lesser degree, a positive DIF finding are good indicators of future relapse of BP. Recently, Charneux et al[25] determined in a retrospective study that at the time of diagnosis, only 59% of active BP patients produced a positive BP230 ELISA result, while 86% produced a positive BP180 ELISA result. As mentioned above, the BP180 ELISA result is correlated with disease extent, but no relationship has yet been evidenced to exist between the BP230 ELISA result and disease extent at diagnosis or the presence of mucosal involvement. Serum anti-BMZ autoantibodies are more frequently detected by IIF when the BP230 ELISA result was positive. [25] In another study, the sensitivity and specificity of ELISA for the BP230 (BPAG1) and BP180 (BPAG2) were 87% and 88%, respectively; however, the combination of BPAG1 ELISA and BPAG2 ELISA led to 8% and 16% gains in sensitivity, compared to either alone. Moreover, the BPAG2 ELISA values were reported to be more closely correlated with initial extent of BP lesions than were the BPAG1 ELISA values. [26] Kusajima et al[27] demonstrated that the BP180-NC16A ELISA indices correlated with BP disease remission more accurately than IIF titers, and represented a useful tool to not only detect BP disease remission but also to assess the efficacy of BP treatment. Tampoia et al[28] reported that the diagnostic specificity for both tests together was over 98%, and diagnostic sensitivity was 90% and 60% for anti-BP180 and anti-BP230, respectively. Furthermore, IgG anti-BP180 titers in their study were significantly correlated with disease activity. The results of previous studies in other countries using the same ELISA kits as in the current study are summarized in [Table - 7].
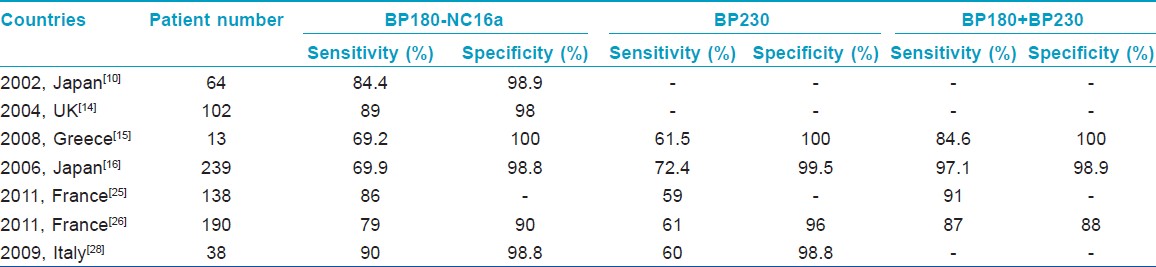
The varying sensitivity of ELISA assay that has been reported by the many studies in the current literature may be attributed to the following reasons: (i) different methods of determining a cut-off value; (ii) differences in the selection and number of the investigated BP sera (for example, the phase of the disease course, or whether or not treatment is ongoing); and (iii) different lengths and sources of recombinant proteins. [14] Yoshida et al[16] confirmed that in the active stage, the BP180-NC16a ELISA is more sensitive than the BP230 ELISA, while in remission, the BP230 ELISA is more sensitive than the BP180-NC16a ELISA. Unfortunately, in our study, the serum-specific concentrations of anti-BP230 antibodies of BP patients in remission stage were not determined and may have provided novel insights into the remission stage of BP.
In this study, 10 of the BP patients yielded negative IIF-SSS results, even though all 10 were positive for BP180 and/or BP230 by IB and 8 were BP180 and/or BP230 positive by ELISA; this may explain the difference in detection sensitivity of our study as compared to others. We also found that the BP180-NC16A ELISA-positive results correlated with BP180 positivity by IB, while the BP230 ELISA-positive results correlated with BP230 positivity by IB. Although a single ELISA diagnostic test is slightly more expensive than IIF in China ($25 vs. $19), we believe that the above-listed advantages of the BP180-NC16A and BP230 ELISA tests justify their use in Chinese clinics.
Conclusion
Using several approaches to assay sera from patients with BP, we reaffirmed that BP180-NC16A ELISA is a useful diagnostic tool for BP in Chinese patients and discovered that BP230 ELISA can enhance the sensitivity of BP diagnosis in this patient population. The combination of BP180-NC16a and BP230 ELISA diagnostic kits represents a feasible BP diagnostic approach since the kits are known to produce reproducible, rapid, and specific results and are technically simple to use and more cost-effective than the IIF-SSS procedure.
| 1. |
Zillikens D. Acquired skin disease of hemidesmosomes. J Dermatol Sci 1999;20:134-54.
[Google Scholar]
|
| 2. |
Moll R, Moll I. Epidermal adhesion molecules and basement membrane components as target structures of autoimmunity. Virchows Arch 1998;432:487-504.
[Google Scholar]
|
| 3. |
Zillikens D, Rose PA, Balding SD, Liu Z, Olague-Marchan M, Diaz LA, et al. Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. J Invest Dermatol 1997;109:573-9.
[Google Scholar]
|
| 4. |
Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, et al. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest 1993;92:2480-8.
[Google Scholar]
|
| 5. |
Liu Z, Sui W, Zhao M, Li Z, Li N, Thresher R, et al. Subepidermal blistering induced by human autoantibodies to BP180 requires innate immune players in a humanized bullous pemphigoid mouse model. J Autoimmun 2008;31:331-8.
[Google Scholar]
|
| 6. |
Hall RP 3rd, Murray JC, McCord MM, Rico MJ, Streilein RD. Rabbits immunized with a peptide encoded for by the 230-kD bullous pemphigoid antigen cDNA develop an enhanced inflammatory response to UVB irradiation: A potential animal model for bullous pemphigoid. J Invest Dermatol 1993;101:9-14.
[Google Scholar]
|
| 7. |
Lazarova Z, Yancey KB. Reactivity of autoantibodies from patients with defined subepidermal bullous diseases against 1 mol/L salt-split skin. Specificity, sensitivity, and practical considerations. J Am Acad Dermatol 1996;35:398-403.
[Google Scholar]
|
| 8. |
Kanitakis J. Indirect immunofluorescence microscopy for the serological diagnosis of autoimmune blistering skin diseases: A review. Clin Dermatol 2001;19:614-21.
[Google Scholar]
|
| 9. |
Pas HH. Immunoblot assay in differential diagnosis of autoimmune blistering skin diseases. Clin Dermatol 2001;19:622-30.
[Google Scholar]
|
| 10. |
Kobayashi M, Amagai M, Kuroda-Kinoshita K, Hashimoto T, Shirakata Y, Hashimoto K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci 2002;30:224-32.
[Google Scholar]
|
| 11. |
Kromminga A, Sitaru C, Hagel C, Herzog S, Zillikens D. Development of an ELISA for the detection of autoantibodies to BP230. Clin Immunol 2004;111:146-52.
[Google Scholar]
|
| 12. |
Gammon WR, Briggaman RA, Inman AO 3 rd , Queen LL, Wheeler CE. Differentiating anti-lamina lucida and anti-sublamina densa anti-BMZ antibodies by indirect immunofluorescence on 1.0 M sodium chloride-separated skin. J Invest Dermatol 1984;82:139-44.
[Google Scholar]
|
| 13. |
Mueller S, Klaus-Kovtun V, Stanley JR. A 230-kD basic protein is the major bullous pemphigoid antigen. J Invest Dermatol 1989;92:33-8.
[Google Scholar]
|
| 14. |
Sakuma-Oyama Y, Powell AM, Oyama N, Albert S, Bhogal BS, Black MM. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol 2004;151:126-31.
[Google Scholar]
|
| 15. |
Patsatsi A, Vyzantiadis TA, Devliotou-Panagiotidou D, Chrysomallis F, Sotiriadis D. Detection of anti-BP180NC16a and anti-BP230 autoantibodies in blister fluid of patients with bullous pemphigoid: The first survey in Greece. Clin Exp Dermatol 2008;33:183-5.
[Google Scholar]
|
| 16. |
Yoshida M, Hamada T, Amagai M, Hashimoto K, Uehara R, Yamaguchi K, et al. Enzyme-linked immunosorbent assay using bacterial recombinant proteins of human BP230 as a diagnostic tool for bullous pemphigoid. J Dermatol Sci 2006;41:21-30.
[Google Scholar]
|
| 17. |
Feng S, Wu Q, Jin P, Lin L, Zhou W, Sang H, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Int J Dermatol 2008;47:225-8.
[Google Scholar]
|
| 18. |
Feng S, Lin L, Jin P, Wu Q, Zhou W, Sang H, et al. Role of BP180NC16a- enzyme-linked immunosorbent assay (ELISA) in the diagnosis of bullous pemphigoid in China. Int J Dermatol 2008;47:24-8.
[Google Scholar]
|
| 19. |
Chang YT, Liu HN, Yu CW, Lin MW, Huang CH, Chen CC, et al. Cytokine gene polymorphisms in bullous pemphigoid in a Chinese population. Br J Dermatol 2006;154:79-84.
[Google Scholar]
|
| 20. |
Schmidt E, Obe K, Bröcker EB, Zillikens D. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol 2000;136:174-8.
[Google Scholar]
|
| 21. |
Tsuji-Abe Y, Akiyama M, Yamanaka Y, Kikuchi T, Sato-Matsumura KC, Shimizu H. Correlation of clinical severity and ELISA indices for the NC16A domain of BP180 measured using BP180 ELISA kit in bullous pemphigoid. J Dermatol Sci 2005;37:145-9.
[Google Scholar]
|
| 22. |
Zillikens D, Mascaro JM, Rose PA, Liu Z, Ewing SM, Caux F, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol 1997;109:679-3.
[Google Scholar]
|
| 23. |
Haase C, Büdinger L, Borradori L, Yee C, Merk HF, Yancey K, et al. Detection of IgG autoantibodies in the sera of patients with bullous and gestational pemphigoid: ELISA studies utilizing a baculovirus-encoded form of bullous pemphigoid antigen 2. J Invest Dermatol 1998;110:282-6.
[Google Scholar]
|
| 24. |
Bernard P, Reguiai Z, Tancrède-Bohin E, Cordel N, Plantin P, Pauwels C, et al. Risk factors for relapse in patients with bullous pemphigoid in clinical remission: A multicenter, prospective, cohort study. Arch Dermatol 2009;145:537-42.
[Google Scholar]
|
| 25. |
Charneux J, Lorin J, Vitry F, Antonicelli F, Reguiai Z, Barbe C, et al. Usefulness of BP230 and BP180-NC16a enzyme-linked immunosorbent assays in the initial diagnosis of bullous pemphigoid: A retrospective study of 138 patients. Arch Dermatol 2011;147:286-91.
[Google Scholar]
|
| 26. |
Roussel A, Benichou J, Randriamanantany ZA, Gilbert D, Drenovska K, Houivet E, et al. Enzyme-linked immunosorbent assay for the combination of bullous pemphigoid antigens 1 and 2 in the diagnosis of bullous pemphigoid. Arch Dermatol 2011;147:293-8.
[Google Scholar]
|
| 27. |
Kusajima E, Akiyama M, Sato M, Natsuga K, Shimizu H. Type XVII collagen ELISA indices significantly decreased after bullous pemphigoid remission. Int J Dermatol 2011;50:238-40.
[Google Scholar]
|
| 28. |
Tampoia M, Lattanzi V, Zucano A, Villalta D, Filotico R, Fontana A, et al. Evaluation of a new ELISA assay for detection of BP230 autoantibodies in bullous pemphigoid. Ann N Y Acad Sci 2009;1173:15-20.
[Google Scholar]
|
Fulltext Views
3,384
PDF downloads
2,643





