Translate this page into:
Exploring the safety and effectiveness of subcutaneous autologous serum therapy versus conventional intramuscular autologous serum therapy in chronic urticaria: An observer-blind, randomized, controlled study
2 Department of Pharmacology, Rampurhat Government Medical College, Rampurhat, West Bengal, India
3 Department of Dermatology, Bankura Sammilani Medical College, Bankura, West Bengal, India
Correspondence Address:
Nilay Kanti Das
Department of Dermatology, Bankura Sammilani Medical College, Kenduadihi, Bankura - 722 102, West Bengal
India
| How to cite this article: Datta A, Chandra S, Saha A, Sil A, Das NK. Exploring the safety and effectiveness of subcutaneous autologous serum therapy versus conventional intramuscular autologous serum therapy in chronic urticaria: An observer-blind, randomized, controlled study. Indian J Dermatol Venereol Leprol 2020;86:632-642 |
Abstract
Background: Autologous serum therapy aims to supplement the existing pharmacotherapy in chronic urticaria by decreasing the antihistamine pill-burden and maintaining symptom-free interval. Subcutaneous autologous serum therapy further modifies the amount of serum (2 mL to 1 mL) and gauge of a needle (24G to 31G) to improve compliance and facilitate ease of application.
Objectives: To assess clinical effectiveness and safety of subcutaneous autologous serum therapy versus conventional intramuscular autologous serum therapy and to compare the quality of life in the two treatment arms.
Methods: Institution-based, assessor-blind, prospective, randomized, parallel-group, active-controlled trial with 32 patients in each treatment arm and analyzed on a modified intention to treat principle. After baseline autologous serum skin test, autologous serum was injected as per randomization every week for 9 consecutive weeks.
Results: Among the study population, conventional intramuscular autologous serum therapy and subcutaneous autologous serum therapy had a comparable duration of disease (P = 0.164, Mann-Whitney U test), autoreactive status (P = 0.796), urticaria total severity score (P = 0.637) and urticaria activity score summed up over 7 days (P = 0.982). Both urticaria activity score summed up over 7 days and total severity score along with antihistamine pill-burden reduced significantly (P < 0.001, Friedman's analysis of variance) in both subcutaneous autologous serum therapy and conventional intramuscular autologous serum therapy from first follow-up onwards (P < 0.05, Post hoc Dunn's test). Significant improvement was noted in patient's as well as physician's global assessment of disease activity improvement scale (P < 0.001, Friedman's analysis of variance). Intergroup analysis showed that there was no significant difference in urticaria activity score summed up over 7 days either at baseline (P = 0.982, Mann-Whitney U test) or at study end (P = 0.398, Mann-Whitney U test). Similar comparable results were found in the total severity score at the end of the study (P = 0.345, Mann-Whitney U test). Dermatology life quality index showed marked improvement with both types of treatment (P < 0.0001, Wilcoxon test), and the intergroup comparison showed comparable dermatology life quality index values (P = 0.994, Mann-Whitney U test). The pain score at the injection site was more with conventional intramuscular autologous serum therapy than subcutaneous autologous serum therapy (P = 0.0115, Mann-Whitney test). Younger age and lower baseline total severity scores were associated with a better therapeutic response. Baseline urticaria activity score added up over a period of 7 days and total severity scores and the diameter of lesions showed a positive correlation with response pattern.
Limitation: Basophil histamine release assay not done. Logistics could not support follow-up beyond the end of treatment.
Conclusion: Subcutaneous autologous serum therapy is not inferior to conventional intramuscular autologous serum therapy with the additional advantage of less pain and operational feasibility.
Introduction
Chronic urticaria is a common and distressing dermatosis that can be defined as daily or almost daily occurrence of widespread itchy wheals for the duration of 6 or more weeks.[1] The primary aim of the treatment of chronic urticaria is symptomatic relief with antihistamines and avoidance of triggering factors. According to the EAACI/GALEN/EDF/WAO guidelines for the management of urticaria, the nonsedating antihistamines are the agents of choice, the dosage of which can be increased up to four times in case of non-responsiveness with 2 weeks of therapy, but that adds to the pill-burden and thus has the potential of noncompliance.[2] Alternative methods include combinations with H2 antihistamines or leukotriene receptor antagonists. Immunosuppressive therapy with cyclosporine, omalizumab, corticosteroids may also be used; however, their use is limited owing to their side-effect profile (s) and/or their prohibitory cost.[3]
In the search for newer modalities to supplement the pharmacotherapy for urticaria, the age-old practice of autologous whole blood therapy can be explored to reduce the burden of pills while maintaining a symptom-free interval which is more relevant in persons not responding to a single daily dose of antihistamine. Available data from India shows autologous serum therapy to be effective in a significant number of patients in maintaining a disease-free interval. Bajaj et al. showed the effectiveness of autologous serum therapy in both autoreactive as well as non-autoreactive patients with chronic urticaria which was further established to be an effective adjuvant therapy in chronic urticaria patients by Debabarman et al.[4],[5] Conventional intramuscular autologous serum therapy involves an intramuscular injection of 2 mL of autologous serum using a 24G needle, whereas subcutaneous injection of 1 mL of autologous serum using a 31G needle is a modification in the conventional method. The present study was a modest attempt to lessen the operational difficulty by reducing the amount of serum (from 2 mL to 1 mL) and using a slender needle (31G instead of 24G) to improve compliance by reducing the pain and discomfort in the patient.
With this background, the present trial was conducted to compare the clinical effectiveness of subcutaneous autologous serum therapy versus conventional intramuscular serum therapy in cases of chronic urticaria who were not responding to a single daily dose of antihistamine and also to compare the safety and quality of life in both treatment groups.
Methods
The study was designed as an institution-based, investigator-blind, randomized, parallel-group, active-controlled clinical trial. Prior clearance was obtained from the institutional ethics committee before the commencement of the study and written informed consent was obtained from each trial participant. The trial was registered prospectively with the Clinical Trial Registry—India bearing registration no. CTRI/2016/05/006907. Adult patients (≥18 years) attending the outpatient department of dermatology of Medical College, Kolkata, West Bengal with chronic urticaria not responding to a single daily dose of antihistamines (10 mg cetirizine) were included as trial participants. Pregnant and lactating women, immunocompromised individuals, those with angioedema and with concomitant systemic illness were excluded from the study.
Sample size
The target sample size was 64, with 32 evaluable subjects in each group. The sample size was calculated as the difference between two means, to detect a difference of 3.5 in total severity score between the groups, with 80% power and 5% probability of type 1 error, assuming a standard deviation of 4.5 and 5 for each group for this parameter and considering possible 10% dropouts.[5]
Visits
The study was carried out for 12 months. Each study participant was given treatment (intramuscular or subcutaneous injections of autologous serum) at weekly intervals for 9 weeks. The first patient was recruited in May 2016 and recruitment was carried out till February 2017.
Screening visit (−3 days)
Patients clinically diagnosed to be having chronic urticaria not responding to a single daily dose of antihistamines (cetirizine 10 mg once daily for 2 weeks) were screened and included according to the inclusion criteria. Informed consent was obtained and they were randomized into treatment groups using a computer-generated randomization sequence with an allocation ratio 1:1. All medications including antihistamines were withdrawn 48 h before the commencement of the study, that is, the patient received autologous serum therapy after a drug-free interval of 48 h.
Baseline visit (Day 0)
The effectiveness parameters at presentation were recorded in a standardized case record form and the dermatology life quality index was filled up.
Autologous serum skin test was performed on each study subject.[7] 0.05 mL of autologous serum was injected immediately intradermally into the patients' left flexor forearm 2 inches below the antecubital crease and 0.05 mL sterile normal saline as negative control into right forearm using 31G sterile disposable 1 mL insulin syringe. The reading of the wheal was taken after 30 min. The autologous serum skin test was considered positive if the diameter of serum-induced wheal was at least 1.5 mm greater than that induced by saline.
Preparation of autologous serum: 5 mL venous blood of the patient was drawn with a sterile, disposable syringe from the antecubital vein in a sterile vacutainer for serum collection. After a holding period of 1 h, the blood was centrifuged at the rate of 2000 revolutions per minute for 10 min at room temperature.
Autologous serum was injected over the lateral aspect of the patients' right arm ( first dose). The autologous serum was given as 1 mL subcutaneously over the lateral aspect of the upper arm using an insulin syringe with a 31G needle in one group and as 2 mL intramuscularly in the deltoid using a 24G needle in the other group.
Cetirizine tablets (10mg) were provided to patients in both treatment groups and were asked to consume them as and when required. They were asked to come back for weekly follow-ups for eight more visits.
Follow-up visits
At each subsequent visit, study parameters were noted, along with adverse effects (if any) and each subject was administered autologous serum therapy according to randomization.
Randomization and blinding
The participants were randomized into each treatment arm using a computer-generated randomization sequence using the WINPEPI software with an allocation ratio 1:1 (unstratified). Allocation concealment was done using sequentially numbered opaque sealed envelopes. Enrolment of the participant was done by the principal investigator who also assigned them into groups following the concealed randomization sequence. Assessor blinding was done by engaging a clinician not involved in randomization for giving intramuscular or subcutaneous autologous serum therapy and recording the effectiveness and safety parameters. This ensured the non-biased recording of the study parameters.
Effectiveness parameters
The primary outcome measures were urticaria total severity score and the urticaria activity score summed up over a period of 7 days. Subjective assessment of symptom improvement was noted using the physician's global assessment of disease activity improvement and patient's global assessment of disease activity improvement using a 5-point Likert scale where 0 indicated no improvement and 5 denoted excellent improvement.
Urticaria activity score for each day denoted the sum of the wheal number score and itch severity score (0–6). Urticaria activity score added up over 7 days represented summation of wheal number score and itch severity scores measured for 7 days ranging from a value from 0 to 42.[8] A wheal score of 0 was assigned when less than 10 small wheals (diameter <3 cm) were present, presence of 10–50 small wheals or less than 10 large wheals (diameter >3 cm) was denoted by score 1. A score of 2 was assigned when more than 50 small wheals or 10 to 50 large wheals were present. A score of 3 denoted wheals covering almost the entire body surface area. Itch severity score ranged between 0 and 3, with score 0 denoting no itch at all, and score 3 depicting severe/intense itching disturbing and hampering daytime activities and sleep. Mild itching was given a score of 1, and moderate itching with score 2 was disturbing but not hampering daytime activities/sleep.
Urticaria total severity score is the summation of each parameter as denoted in [Table - 1].[4] It was calculated using the sum of six parameters namely the number of wheals, size of wheals, intensity of pruritus, duration of persistence of symptoms, frequency of appearance, and frequency of antihistamine use. Each parameter score ranged from 0 to 3 [Table - 1].
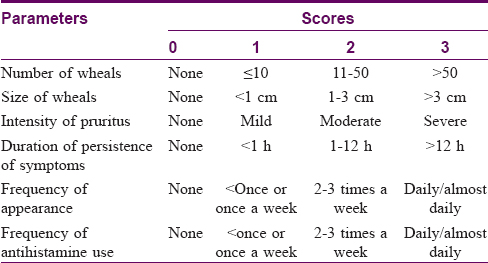
Overall disease severity was classified as:
Clear (total severity score = 0), mild (total severity score 1–6), moderate (total severity score 7–12), severe (total severity score 13–18).
Our operational definitions for a response to therapy were as follows:
Complete responder = No antihistamine requirement
Moderate responder = 1–3 tablets of antihistamine/week
Non-responder = Daily or almost daily requirement of antihistamines
Quality of life in patients with chronic urticaria was assessed by a validated vernacular version of the dermatology life quality index [https://www.cardiff.ac.uk/medicine/resources/quality-of-life-questionnaires/dermatology-life-quality-index], which consists of 10 questions, each scored between 0 and 3, used after permission of the developer.
Safety parameters
Spontaneously reported adverse events were recorded at each follow-up. Changes in laboratory measures of routine hemogram, serum urea, creatinine, liver function tests were noted at baseline and at the end of 9 weeks.
Tolerability parameters
The pain was assessed on a 5-point Likert scale and was assessed by participants just after the injection. Score 5 was given to maximum pain and 0 to no pain.
Statistical analysis
Descriptive statistics were expressed as ratio, percentage and proportion for categorical data and mean ± SD, median, interquartile range for numerical data. The normality of distribution was checked by the D'Agostino-Pearson test and parametric data were analyzed using students' t-test, analysis of variance test. For nonparametric data as in urticaria activity score summed up over 7 days, total severity score, physicians' and patients' global assessment of disease activity improvement, dermatology life quality index, Mann-Whitney's test, Wilcoxon rank-sum test, Friedman's analysis of variance with post hoc Dunn's test and Kruskal-Wallis test (as applicable) were done. The homogeneity of the population was tested by a variance ratio test (F test). Categorical data were analyzed by using the Chi-square test and Fisher's exact test (as applicable). The statistical software Medcalc® v 12.5.0.0 was used for analysis and Graph-pad Prism was used for drawing the randomization table. Modified intention to treat analysis was done. P > 0.05 was considered to be statistically significant.
Results
Among the 93 study participants screened, 64 were recruited and randomized into two intervention groups [Figure - 1] shows the flow of study participants.
 |
| Figure 1: Flowchart and profile of the study population |
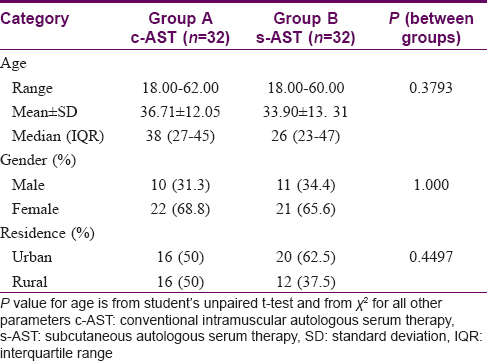
[Table - 2] denotes the demographic profile of the study population and the study groups were found to be comparable with respect to age, gender distribution, area of residence, socioeconomic status, literacy status and occupation. There was a predominance of women in both the study groups, with housewives comprising the bulk of study participants. Most patients were literate (55; 85.9%) and had completed at least their primary education, and were above the poverty line (53; 82.8%).
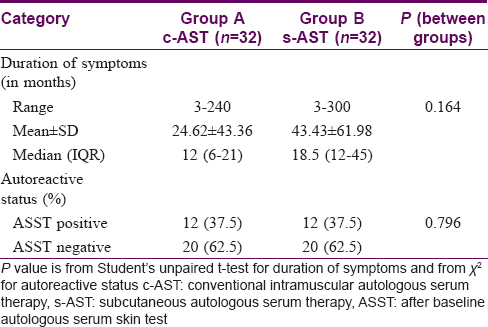
No intergroup variation was noticed in the baseline clinical profile of the study participants with respect to disease duration and autoreactivity status [Table - 3].
Forty seven (73.4%) out of 64 study participants reported some physical agents that aggravated their wheals, with multiple factors sometimes present in a single individual. Physical pressure was found to be the major aggravating factor in conventional intramuscular autologous serum therapy group (10; 31.2%) whereas sun exposure (11; 34.3%) and exercise (12; 37.5%) were predominant in the subcutaneous autologous serum therapy group. Dermographism was present in only 13 (20.3%) study participants. Both the groups were comparable with regard to aggravating factors, associated systemic features and coexisting illness (P > 0.05, Chi-square test).
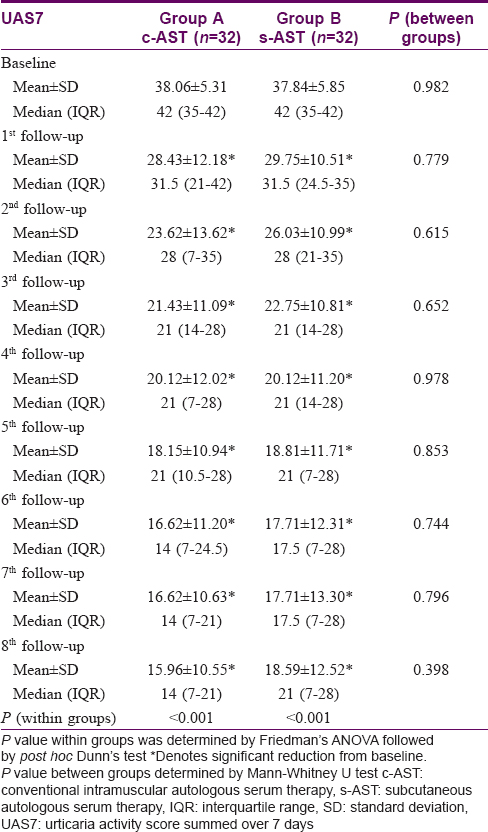
Urticaria activity score summed for 7 days showed a reduction from mean baseline score 37.84 ± 5.85 to mean end-of-treatment score 18.59 ± 12.52 in individuals receiving conventional intramuscular autologous serum therapy. Similarly, trial participants receiving subcutaneous autologous serum therapy showed a reduction from a baseline score of 38.06 ± 5.31 to a score of 15.96 ± 10.55 at the end of treatment. Both groups showed a consistent reduction in urticaria activity score summed over 7 days from the baseline and this significant trend was noted from the first follow-up onwards (Friedman's analysis of variance < 0.001). However, the statistical analysis failed to reveal any intergroup variation, neither at baseline nor at any subsequent follow-ups [Table - 4].
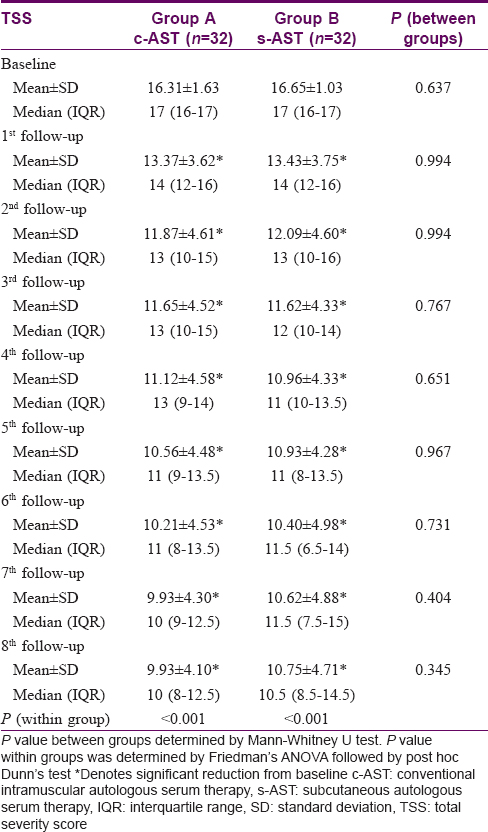
Urticaria total severity score reduced from 16.31 ± 1.63 to 9.93 ± 4.10 in the conventional intramuscular autologous serum therapy group and 16.65 ± 1.03 to 10.75 ± 4.71 in subcutaneous autologous serum therapy group at baseline and at end of treatment, respectively. Both groups showed a consistent reduction in total severity score from the baseline and this significant trend was noted from the first follow-up onwards (Friedman's analysis of variance < 0.001). However, the statistical analysis failed to reveal any significant intergroup variation, neither at baseline nor at any subsequent follow-ups [Table - 5].
The opinion of patients regarding their baseline disease severity (patient's global assessment of disease activity improvement score) did not vary in between the two treatment groups. However, their response to treatment was reflected in their assessment of disease severity in subsequent follow-ups, with a consistent improvement in both treatment groups. Statistical analysis with Friedman's analysis of variance with post hoc Dunn's test revealed significant changes evident from first follow-up onwards (P < 0.001). Despite a visible difference in the line diagrams, at no follow-up whatsoever any significant intergroup variation was observed [Figure - 2].
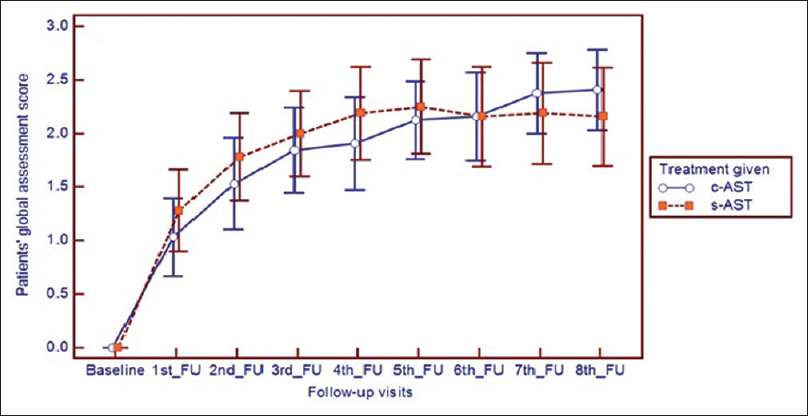 |
| Figure 2: Improvement in patient global assessment scale in both treatment groups, where improvement is noted from 1st follow-up onwards |
Assessment of the disease severity by the physician showed that at baseline the disease was severe (Physicians' global assessment of disease activity improvement scale value, 0) in both the treatment groups. However, in subsequent visits, with therapy, the disease severity significantly reduced in individual treatment groups (P < 0.001). Individual follow-ups when compared to baseline, varied significantly (P < 0.001) from the first follow-up onwards. Statistical analysis revealed no significant intergroup variation [Figure - 3].
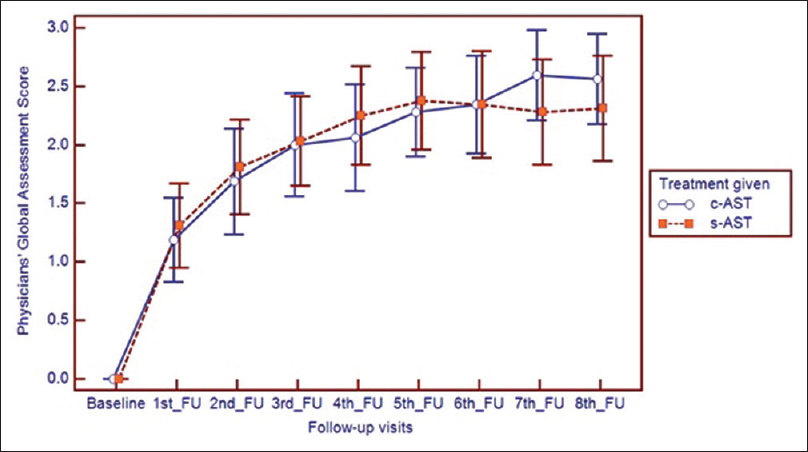 |
| Figure 3: Change in physician global assessment scale in both treatment groups with improvement evident from 1st follow-up onwards |
The study population comprised chronic urticaria patients requiring more than once-daily dosing of antihistamines for control of symptoms. At baseline, the mean number of antihistamines taken in the last 7 days was 12.06 (± 4.17) in the conventional intramuscular autologous serum therapy group and 13.31 (± 6.19) in a subcutaneous autologous serum therapy group. With therapy, the antihistamine requirement reduced in both groups significantly from the first follow-up onwards. At the eighth follow-up, mean antihistamine requirement had decreased to 4.03 (± 4.43) and 6.31 (± 6.58) in the conventional intramuscular autologous serum therapy group and subcutaneous autologous serum therapy group, respectively. The total number of tablets of antihistamines taken in a week was, however, comparable in both groups at baseline and also in subsequent follow-ups. There was no significant intergroup difference in antihistamine usage at the end of 8 weeks (P = 0.182).
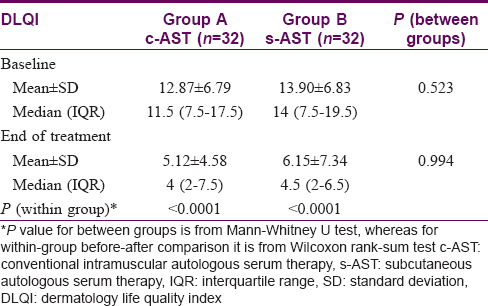
The quality of life (assessed by dermatology life quality index) was comparable (P = 0.523) in both the treatment arms at baseline as shown in [Table - 6]. The dermatology life quality index improved significantly at the end of the study with both conventional intramuscular autologous serum therapy (P < 0.0001) and subcutaneous autologous serum therapy (P < 0.0001) and intergroup comparison showed no difference (P = 0.994).
During the trial, 19 (29.7%) study participants complained of some adverse events, most common being pain at the injection site. Two of them complained of dryness of mouth and one developed constipation. All of these were level I according to Hartwig's severity assessment scale and none of them required stoppage of trial therapy. Pain at the injection site was significantly more with conventional intramuscular autologous serum therapy than subcutaneous autologous serum therapy (P = 0.0115) with mean pain score 2.00 ± 2.03 in the conventional intramuscular autologous serum therapy group and 0.71 ± 1.14 in the subcutaneous autologous serum therapy group.
Both autoreactive and non-autoreactive subpopulations showed marked improvement in urticaria activity score summed over 7 days and total severity score with autologous serum therapy, irrespective of the mode of administration. (P < 0.001) The urticaria activity score added up over 7 days and the total severity score showed no difference between autoreactive and non-autoreactive urticaria at baseline or at any of the follow-ups [Figure - 4] and [Figure - 5].
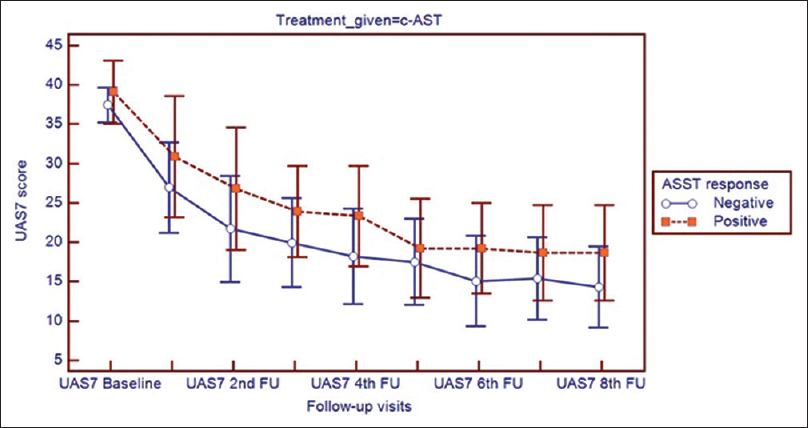 |
| Figure 4: Improvement of urticaria activity score summed over 7 days score in participants receiving conventional intramuscular autologous serum therapy in subgroups showing autologous serum skin test response positive (autologous serum skin test = 1) and response negative (autologous serum skin test = 0) |
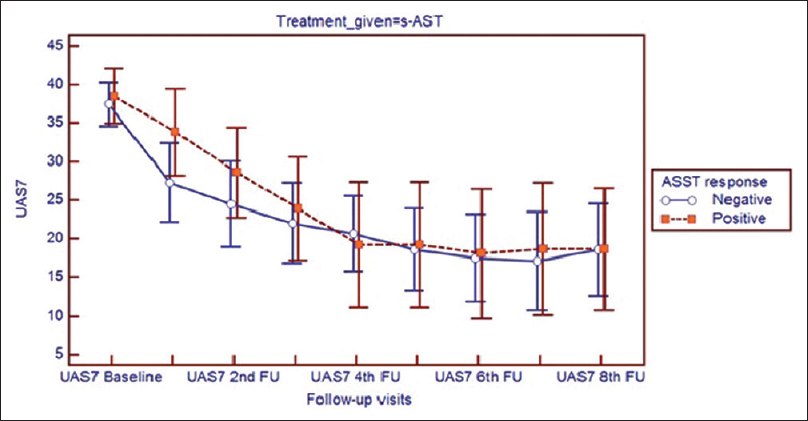 |
| Figure 5: Improvement of urticaria activity score summed over 7 days score in participants receiving subcutaneous autologous serum therapy in subgroups showing autologous serum skin test response positive (autologous serum skin test = 1) and response negative (autologous serum skin test = 0) |
There was no significant effect of any subtype of urticaria on any of the effectiveness parameters. Both urticaria activity score and total severity scores were comparable at baseline and at end of treatment.
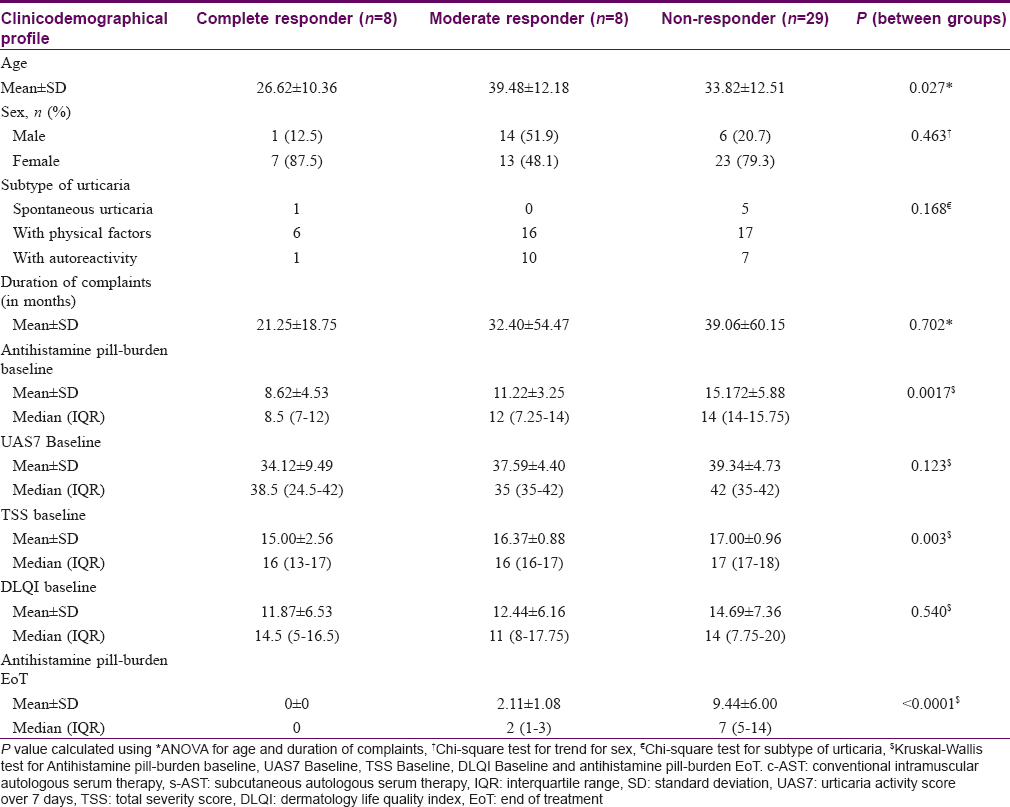
Out of 64 trial participants, eight (12.5%) were complete responders with no requirement of antihistamine in 7 days, 27 (42.2%) were moderate responders with antihistamine requirement up to thrice a week and 29 (45.3%) were non-responders with a daily or almost daily requirement of antihistamines. [Table - 7] depicts the clinicodemographical profile of the study population according to the type of response along with the antihistamine pill-burden at baseline and at end of treatment. Analysis of variance revealed age as a significant factor in determining response to therapy, where younger age was associated with a better response (P = 0.027). A lower value of the total severity score at baseline was also associated with a better response (P = 0.003, Kruskal-Wallis test). Gender of individual, any subtype of urticaria, duration of complaints, baseline scores of urticaria activity and dermatology life quality index were found to have no significant value in predicting therapeutic response. A significant difference was noted in the antihistamine pill-burden between the groups both at baseline (P = 0.0017, Kruskal-Wallis test) and also at end of treatment (P=< 0.0001, Kruskal-Wallis test). Logistic regression by linear model found that age, gender, duration of illness, urticaria score (either urticaria activity score/total severity score) at baseline, either form of autologous serum therapy (subcutaneous or intramuscular) does not influence the response outcome (non-responder/complete responder) of the treatment (P = 0.061, Hosmer and Lemeshow test for goodness of fit).
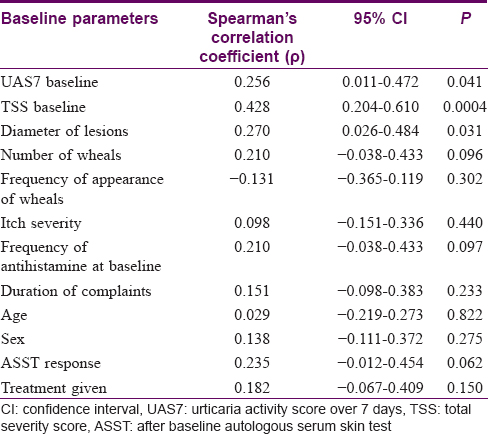
[Table - 8] depicts the correlation of clinicodemographic parameters with the response rate. The type of responder was graded into 1 = complete response, 2 = moderate response, 3 = non-responder. Spearman's rank correlation shows that the baseline urticaria activity score summed over 7 days is positively correlated with the type of responder (rho = 0.256). The baseline total severity score also showed a positive correlation with the response pattern (rho = 0.428). Of the various determinants of urticaria activity score summed up over 7 days and total severity score, the diameter of the urticaria lesions showed a positive correlation (rho = 0.270) with response grade. However, the other determinants showed a not so strong correlation with response grade.
Discussion
Urticaria has been recognized and ascribed many names since the days of Hippocrates. (4th century BC) Similarly, an array of medications including autologous whole blood injections have been tried to curb the symptoms of urticaria.[9] With the discovery of antihistamines by Bovet and Staub in 1937, the practice of autohemotherapy in chronic urticaria gradually fell into disuse. But a sizable proportion of patients in the urticaria spectrum develop symptoms even while on antihistamines. Thus, in pursuit of other treatment modalities and also to tackle the increasing pill-burden and poor quality of life arising out of the disease as well as treatment-related side effects, renewed interest has been generated in this old therapeutic modality. Various studies have proven the efficacy of autohemotherapy in chronic urticaria which was further refined to autologous serum therapy. Over a period of time the operational limitation of conventional autologous serum therapy required refinement [Table - 9] and [Table - 10].[10],[11],[12],[16]
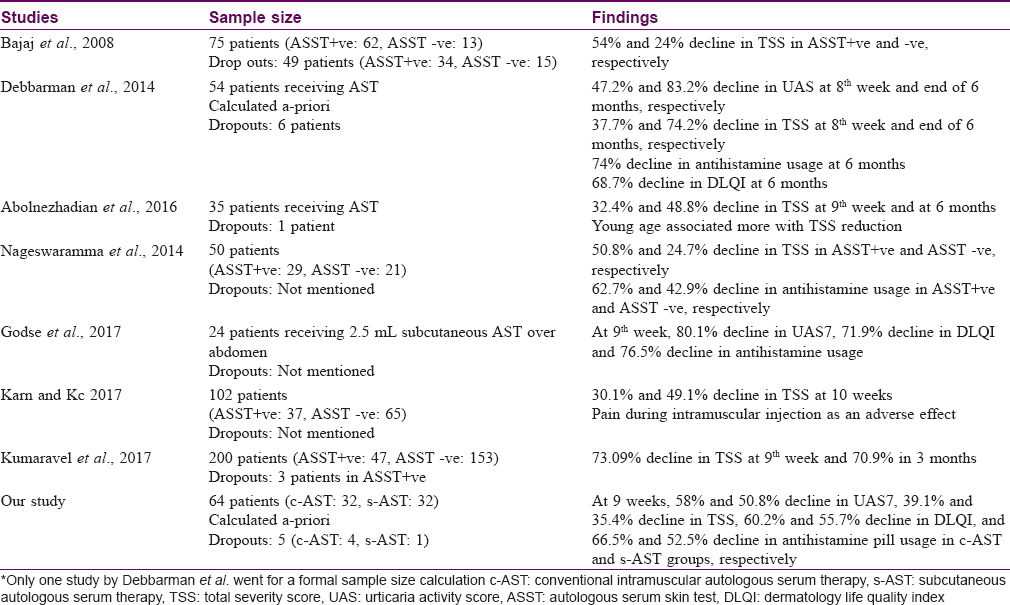
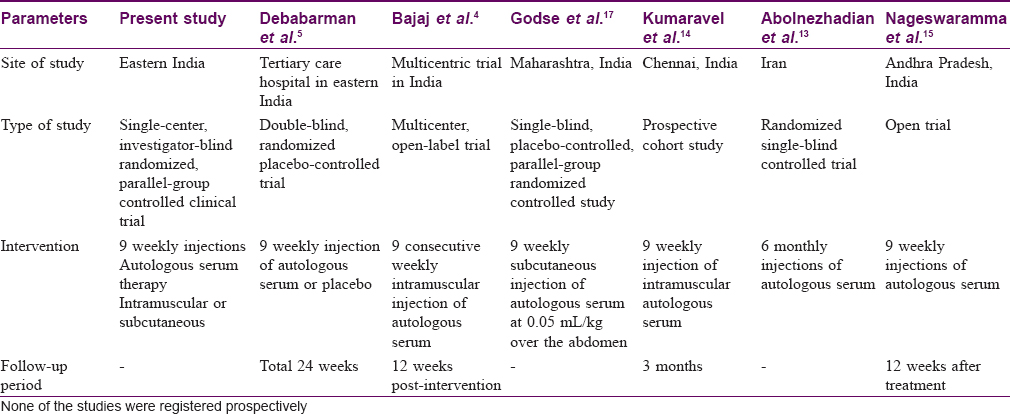
The present study intended to compare the effectiveness and safety of conventional intramuscular and subcutaneous autologous serum therapy in chronic urticaria unresponsive to a single daily dose of 10 mg cetirizine. The effectiveness parameters urticaria activity score over 7 days and urticaria total severity score showed a significant reduction from baseline in each of the subsequent follow-ups in study participants treated with the conventional intramuscular autologous serum therapy as well as subcutaneous autologous serum therapy. This was clinically evident as decrease in both number and size of wheals, from first follow-up onwards. Besides decrease in urticaria symptoms, there was also decrease in duration, severity, frequency of appearance of wheals, as well as in the frequency of antihistamine intake in both the treatment groups. This was observed in conjunction with a marked reduction in the antihistamine pill-burden at the study end. The relief obtained in both the treatment groups is remarkable and both are optimistic modalities of second-line therapeutic agents in chronic urticaria. Since intergroup analysis showed comparable results, thus subcutaneous autologous serum therapy is as effective as conventional intramuscular autologous serum therapy and the upgradation in terms of operational feasibility and patient compliance is an additional advantage without compromising on the therapeutic efficacy.
Chronic urticaria severely interferes with quality of life and also impairs daily activities including personal care, sleep, rest, work, even sexual activities.[6] The decline in dermatology life quality index as evident in the present study, as well as previous studies, indicates that autologous serum therapy in its upgraded form (subcutaneous autologous serum therapy) adds to the therapeutic armamentarium and offers the patients a better quality of life with symptom control [Table - 9].
One of the important goals of our study was to bring about a decline in the antihistamine pill-burden, thereby lessening side effects and also the expenditure associated with medications. It underwent a definite decline with 9 weekly injections of both intramuscular and subcutaneous autologous serum therapy. At baseline, all study participants had the daily requirement of antihistamines for symptom control. With therapy, this was reduced to no antihistamine requirement in 8 participants (12.5%), once a week antihistamine in 7 study participants (10.9%) and antihistamine requirement twice or thrice a week in 20 participants (31.2%). Among the rest, 29 (45.3%) study participants who required daily or almost daily antihistamines for control of symptoms, 25% had a reduction in the number of tablets of antihistamines required in a week. A significant difference, however, was noted both at baseline and at end of treatment between complete responders, moderate responders and non-responders with regards to the antihistamine pill-burden. A similar decline has been noted in previous studies [Table - 9].
Correlation study, however, revealed baseline scores of urticaria activity score summed over 7 days, total severity score and diameter of wheals to have a positive correlation with response grade [Table - 8]. It was noted that the higher the value of the urticaria activity score summed over 7 days and total severity score at baseline, the response grade was more toward incomplete response. Also, more the diameter of the urticaria lesions, less complete was the response. Thus, a higher grade of disease showed a poorer response, and thus, while selecting patients for autologous serum therapy these prognostic indicators need to be kept in mind. Regarding autoreactive status, the result is conflicting with some studies finding a better response in those with autoreactive urticaria while others, like ours, found beneficial effect in both subsets.[4],[5],[14]
The baseline score of the total severity score appears to be a decisive factor in predicting response to therapy (P = 0.003). Total severity score is a comprehensive score that computes the number and size of wheals as well as the frequency and severity of symptoms along with antihistamine requirement. Regardless of the duration of chronic urticaria, response to therapy seems to be related to the frequency of appearance of symptoms, severity and duration of persistence of symptoms, baseline requirement of antihistamines along with the number and size of wheals. Thus computing the total severity score before initiating autologous therapy could save both patient and physician from unnecessary invasive procedures of blood collection and injection.
In a systematic review of autohemotherapy in urticaria, Brewer noted no major adverse effects, and short-lived minor effects similar in frequency to those from placebo injections.[18] In the present study, pain at the injection site was the major side-effect, observed more with conventional intramuscular autologous serum therapy but was found to be significantly less with subcutaneous autologous serum therapy (P = 0.0115). The reduced pain severity with subcutaneous autologous serum therapy is of marked benefit over conventional intramuscular autologous serum therapy by reducing patient discomfort, thereby ensuring treatment adherence and compliance. Though it was a minor adverse effect (Level I on Hartwig's severity assessment scale) and did not require cessation of therapy, less pain is welcomed by the patients receiving it.
The study was not without limitations though. Autoreactivity could not be assessed using the basophil histamine release assay due to lack of facility and we were unable to follow up the patients beyond the end of treatment due to logistic reasons.
Conclusion
Subcutaneous autologous serum therapy is not inferior to conventional intramuscular autologous serum therapy in the treatment of chronic urticaria with the additional advantage of less pain and ease of administration. Thus, it can be an effective adjunct in unresponsive urticaria in alleviating symptoms, reducing the antihistamine pill-burden and ameliorating the quality of life.
Acknowledgment
The authors acknowledge the guidance of Prof. D. Bandyopadhyay, Head of Department and the support obtained from faculty, residents and staff of the department of Dermatology, Medical College, Kolkata.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Greaves MW, Sabroe RA. ABC of allergies. Allergy and the skin. I-Urticaria. BMJ 1998;316:1147-50.
[Google Scholar]
|
| 2. |
Zuberbier T, Asero R, Bindslev-Jensen C, Canonica G, Church MK, Gimenez-Arnau AM, et al. EAACI/GA2LEN/EDF/WAO guideline: Management of urticaria. Allergy 2009;64:1427-43.
[Google Scholar]
|
| 3. |
Ferrer M, Bartra J, Giménez-Arnau A, Jauregui I, Labrador-Horrillo M, Ortiz de Frutos J, et al. Management of urticaria: Not too complicated, not too simple. Clin Exp Allergy 2015;45:731-43.
[Google Scholar]
|
| 4. |
Bajaj AK, Saraswat A, Upadhyay A, Damisetty R, Dhar S. Autologous serum therapy in chronic urticaria: Old wine in a new bottle. Indian J Dermatol Venereol Leprol 2008;74:109-13.
[Google Scholar]
|
| 5. |
Debabarman P, Sil A, Datta PK, Bandyopadhyay D, Das NK. Autologous serum therapyin chronic urticaria: A promising complement to antihistamines. Indian J Dermatol 2014;59:375-82.
[Google Scholar]
|
| 6. |
O'Donell BF, Lawlor F, Simpson J, Morgan M, Greaves MW. The impact of chronic urticaria on the quality of life. Br J Dermatol 1997;136:197-201.
[Google Scholar]
|
| 7. |
Vohra S, Sharma NL, Mahajan VK. Autologous serum skin test: Methodology, interpretation and clinical applications. Indian J Dermatol Venereol Leprol 2009;75:545-8.
[Google Scholar]
|
| 8. |
Hollis K, Proctor C, McBride D, Balp M, McLeod L, Hunter S, et al. Comparison of urticaria activity score over 7 days (UAS7) values obtained from once-daily and twice-daily versions: Results from the ASSURE-CSU study. Am J Clin Dermatol 2018;19:267-74.
[Google Scholar]
|
| 9. |
Nadkarni N. Urticaria: Historical aspects. In: Godse KV, editor. Urticaria. 1st ed.. New Delhi: Jaypee Brothers Medical Publishers; 2016. p. 1-2.
[Google Scholar]
|
| 10. |
Staubach P, Onnen K, Vonend A, Metz M, Siebanhaar F, Tschentscher I, et al. Autologous whole blood injections to patients with chronic urticaria and a positive autologous serum skin test: A placebo-controlled trial. Dermatology 2006;212:150-9.
[Google Scholar]
|
| 11. |
Tseng JT, Lee W, Lin S, Hsu C, Yang H, Wang K, et al. Autologous serum skin test and autologous whole blood injections to patients with chronic Urticaria: A retrospective analysis. Dermatol Sinica 2009;27:27-36.
[Google Scholar]
|
| 12. |
Kocatürk E, Aktaş S, Türkoǧlu Z, Kavala M, Zindanci I, Koc M, et al. Autologous whole blood and autologous serum injections are equally effective as placebo injections in reducing disease activity in patients with chronic spontaneous urticaria: A placebo controlled, randomized, single-blind study. J Dermatolog Treat 2012;23:465-71.
[Google Scholar]
|
| 13. |
Abolnezhadian F, Alyasin S, Amin R, Babaei M. The Effect of Autologous Serum Therapy on Disease Severity in Patients with Chronic Urticaria. Iran J Allergy Asthma Immunol 2016;15:328-33.
[Google Scholar]
|
| 14. |
Kumaravel S, Manjula J, Balamurugan L, Sindhuja SD, Anandan H. Chronic autoimmune urticaria and efficacy of autologous serum therapy. Int J Sci Stud 2017;4:163-6.
[Google Scholar]
|
| 15. |
Nageswaramma S, Sarojini VL, Pujitha BB, Sirisha G, Bindu GS. Efficacy of autologous serum therapy in chronic urticaria. Int J Contemp Med Res 2017;4:110-2.
[Google Scholar]
|
| 16. |
Karn D, Kc S. Clinical otcome of autologous serum therapy in chronic idiopathic urticaria. J Nepal health Res Counc 2017;15:71-4.
[Google Scholar]
|
| 17. |
Godse KV, Nadkarni N, Patil S, Mehta A. Subcutaneous autologous serum therapy in chronic spontaneous urticaria. Indian J Dermatol 2017;62:505-7.
[Google Scholar]
|
| 18. |
Brewer DD. A systematic review of autohemotherapy as a treatment for urticaria and eczema. Cureus 2009;6:e233.
[Google Scholar]
|
Fulltext Views
7,044
PDF downloads
2,434





