Translate this page into:
Faropenem-induced urticarial vasculitis
Corresponding author: Dr. Dibyendu Bikash Bhanja, Department of Dermatology, Venereology, and Leprosy, R. G. Kar Medical College, 1, Khudiram Bose Sarani, Kolkata - 700 004, West Bengal, India. dibyendubhanja0901@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bhanja DB, Sil A, Panigrahi A, Chakraborty S, Datta M. Faropenem-induced urticarial vasculitis. Indian J Dermatol Venereol Leprol 2021;87:146-146.
Sir,
Faropenem is a newer generation broad-spectrum oral b-lactam antibiotic belonging to carbapenem group, commonly used for upper- and lower-respiratory tract, and genitourinary infections.1 Although diarrhea, nausea, and vomiting occur infrequently as side-effects of faropenem, adverse cutaneous reactions are exceedingly rare. Isolated cases of generalized bullous drug eruption and acute generalized exanthematous pustulosis due to faropenem have been reported.2 Herein, we describe a case of faropenem-induced urticarial vasculitis.
A 35-year-old man presented with a 15-day history of bright red, painful, and persistent skin rash over both thighs and legs, associated with joint pain. Prior to the appearance of skin lesions, he was suffering from urinary tract infection for 1 week. Escherichia coli was isolated in urine culture and found to be sensitive to carbapenem group of antibiotic. He had taken faropenem 200 mg thrice daily for 10 days, as prescribed by his treating physician. Skin lesions initially appeared over the right thigh 2 days after initiation of the treatment. But he continued taking the medication for the next 8 days, even though the lesions spread to involve both his lower limbs. There was no history suggestive of any underlying autoimmune connective tissue disorders, malignancy, or family history of similar complaints. Cutaneous examination revealed multiple tender, annular and arciform, partially blanchable, erythematous plaques distributed over the extensor and flexor aspects both thighs and legs [Figure 1]. Purpuric changes were appreciated on diascopy. Complete hemogram was normal except for increased erythrocyte sedimentation rate (30 mm/h, reference range 0–15 mm/h). Liver function tests were normal. Serology for human immunodeficiency virus, viral hepatitis markers and rapid plasma reagin test were normal. Results for antinuclear antibodies, antineutrophil cytoplasmic antibodies, cryoglobulins and rheumatoid factor serology were negative. Serum protein electrophoresis and complement levels were normal. Histopathological examination from erythematous margin revealed acanthosis, dermal edema, lymphocytic, neutrophilic and eosinophilic infiltration of the vessel wall with endothelial cell damage and erythrocyte extravasation, suggestive of urticarial vasculitis [Figure 2]. The causal relationship between faropenem and urticarial vasculitis was found to be “probable” according to the objective causality assessment by the Naranjo adverse drug reaction probability scale (Naranjo score = 6) [Table 1]. As per the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) criteria, the assigned causality category for this adverse drug reaction was revealed as “probable/likely.” Based on the suggestive history, clinical features, and histopathological examination, a diagnosis of faropenem-induced urticarial vasculitis was considered. The patient was treated with oral prednisolone (40mg/day) for 7 days along with a nonsedating antihistaminic drug (fexofenadine), which resulted in complete resolution of lesions within a week. There was no recurrence observed on regular follow-up over 6 months.
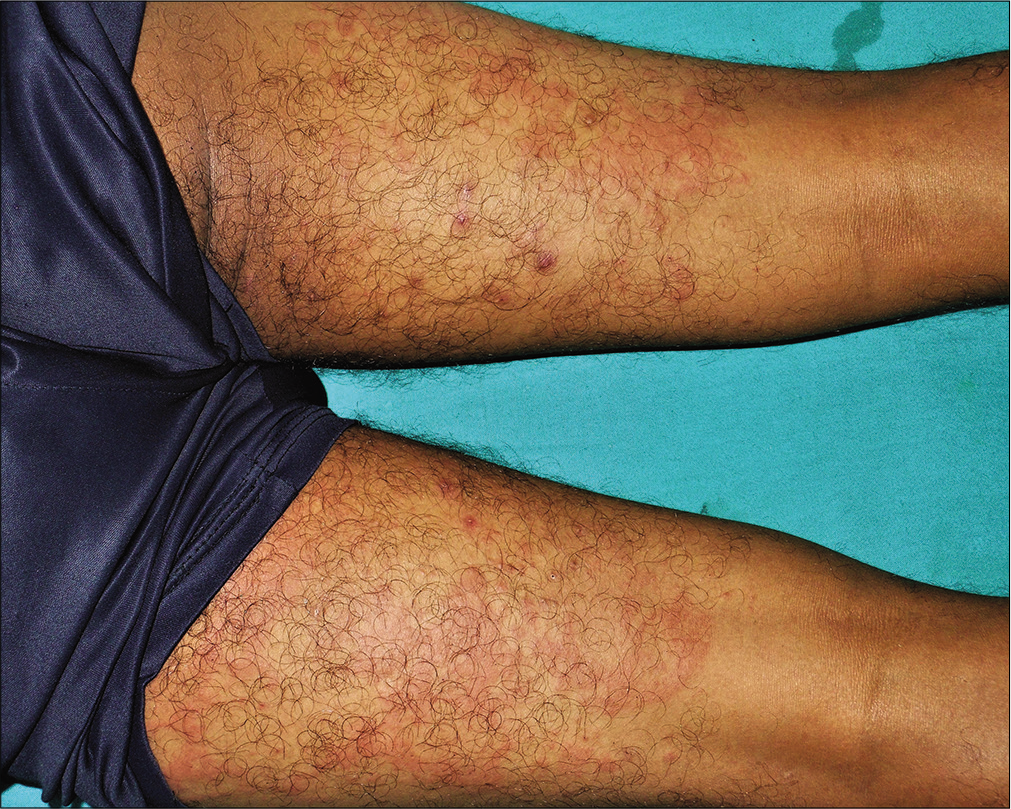
- Multiple tender, annular and arciform, partially blanchable, erythematous plaques distributed over the posterior aspects of both thighs
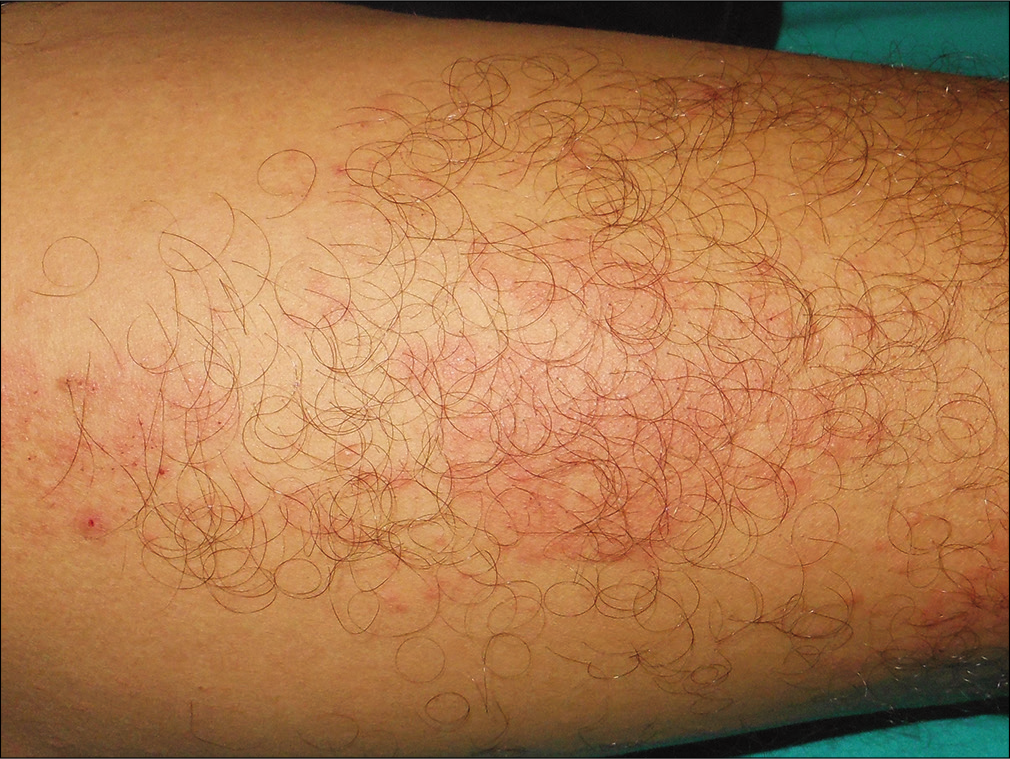
- Anterior aspect of right thigh
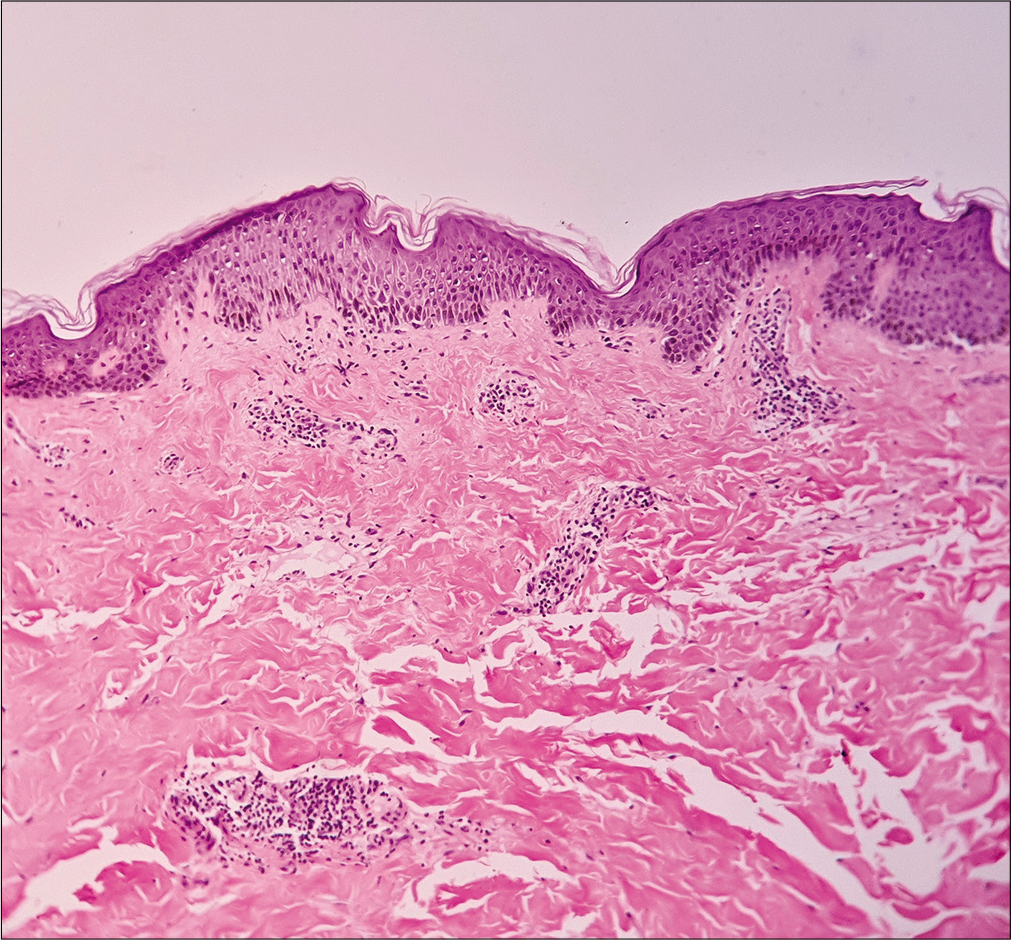
- Acanthosis, dermal edema, and perivascular inflammatory cell infiltrate in papillary dermis (H and E, ×100)
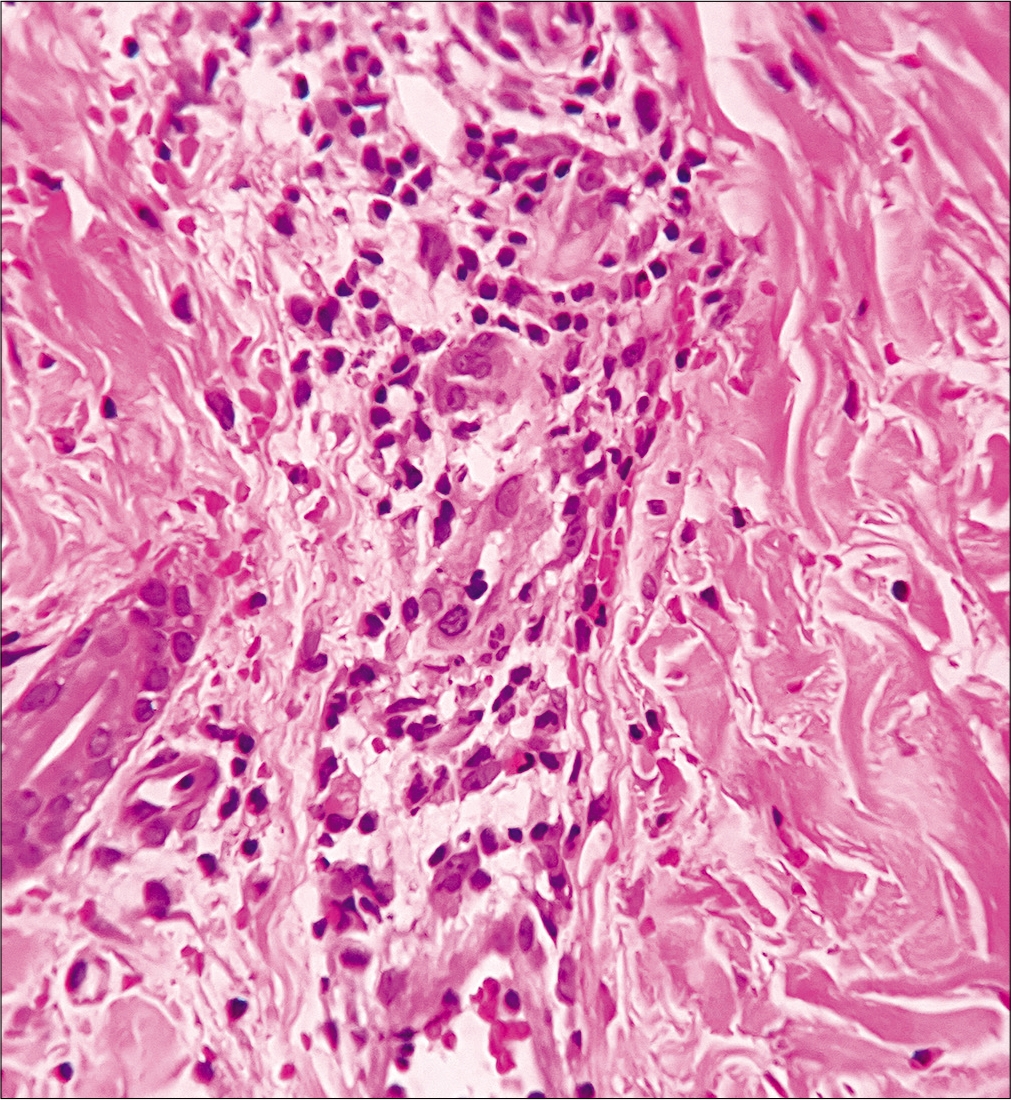
- Lymphocytic, neutrophilic and few eosinophilic infiltrations of the vessel wall with endothelial cell damage, and erythrocyte extravasation suggestive of urticarial vasculitis. (H and E, ×400)
| Question | Yes | No | Do not know | Score |
|---|---|---|---|---|
| 1. Are there previous conclusive reports on this reaction? | +1 | 0 | 0 | 0 |
| 2. Did the adverse event appear after the suspected drug was administered? | +2 | −1 | 0 | +2 |
| 3. Did the adverse event improve when the drug was discontinued or a specific antagonist was administered? | +1 | 0 | 0 | +1 |
| 4. Did the adverse event reappear when the drug was re-administered? | +2 | −1 | 0 | 0 |
| 5. Are there alternative causes that could on their own have caused the reaction? | −1 | +2 | 0 | +2 |
| 6. Did the reaction reappear when a placebo was given? | −1 | +1 | 0 | 0 |
| 7. Was the drug detected in blood or other fluids in concentrations known to be toxic? | +1 | 0 | 0 | 0 |
| 8. Was the reaction more severe when the dose was increased or less severe when the dose was decreased? | +1 | 0 | 0 | 0 |
| 9. Did the patient have a similar reaction to the same or similar drugs in any previous exposure? | +1 | 0 | 0 | 0 |
| 10. Was the adverse event confirmed by any objective evidence? | +1 | 0 | 0 | +1 |
| Total score | 6 |
Score ≥9=definite ADR, 5-8=probable ADR, 1-4=possible ADR, and 0=doubtful ADR. ADR: Adverse drug reaction
Urticarial vasculitis is a distinctive type of small vessel vasculitis clinically characterized by recurrent episodes of painful, persistent urticarial lesions that demonstrate the histopathologic features of leukocytoclastic vasculitis. Urticarial vasculitis with normal complement levels are diagnosed to have idiopathic leukocytoclastic vasculitis, which is usually limited to skin and self resolving. Patients with hypocomplementemic urticarial vasculitis are more likely to have systemic involvement.3
Apart from the association with systemic lupus erythematosus, urticarial vasculitis may be associated with other autoimmune disorders (Sjogren’s syndrome), serum sickness, cryoglobulinemia, infections, certain medications (cimetidine, diltiazem, potassium iodide, fluoxetine, nonsteroidal inflammatory drugs, glatiramer acetate, levetiracetam, enalapril, telmisartan), and hematologic malignancies (plasma cell dyscrasias, leukemias).4,5 While evaluating a case of urticarial vasculitis, differential diagnosis of urticaria, atypical erythema multiforme, serum sickness-like reactions, Schnitzler syndrome and adult-onset Still’s disease should be considered and ruled out. In our patient, a comprehensive workup was performed which ruled out an underlying autoimmune disorder, infection, malignancy, and other systemic diseases. Faropenem is the most likely culprit, as evidenced by Naranjo probability scale and WHO-UMC criteria.
Faropenem-induced urticarial vasculitis is hitherto unreported in the literature (after extensive search in PubMed and Medline databases). In conclusion, this case is being reported for its rarity and also to create awareness among practicing physicians about the possibility of encountering such an adverse cutaneous reaction with this commonly prescribed medication.
Acknowledgement
The authors would like to thank the patient for his co-operation during hospital visit and in providing consent for the clinical photograph.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
References
- Generalized Bullous Drug Eruption to Faropenem-Hitherto Unreported. Indian J Dermatol. 2017;62:101-3.
- [CrossRef] [PubMed] [Google Scholar]
- Acute generalized exanthematous pustulosis caused by Faropenem: A possible pathogenetic role for interleukin-23. Acta Derm Venereol. 2016;96:265-6.
- [CrossRef] [PubMed] [Google Scholar]
- Urticarial vasculitis and hypocomplementemic urticarial vasculitis syndrome. Immunol Allergy Clin North Am. 2004;24:183-213.vi.
- [CrossRef] [PubMed] [Google Scholar]
- Hypocomplementemic urticarial vasculitis: A rare presentation of systemic lupus erythematosus. Int J Dermatol. 2006;45:1057-61.
- [CrossRef] [PubMed] [Google Scholar]
- Enalapril induced normocomplementemic urticarial vasculitis. Indian J Dermatol Venereol Leprol. 2015;81:73-4.
- [CrossRef] [PubMed] [Google Scholar]





