Translate this page into:
Fatal outcome of DRESS syndrome associated with esomeprazole
2 National Center of Pharmacovigilance, 9 Avenue du Dr Zouhaier Essafi 1006, Tunis, Tunisia
3 Tunis El Manar University, Medicine Faculty, 15 Rue Djebel Lakhdhar, La Rabta, 1007, Tunis; Department of Dermatology, Habib Thameur hospital, Tunis, Tunisia
Correspondence Address:
Ahmed Za�em
National Center of Pharmacovigilance, 9 Avenue du Dr Zouhaier Essafi 1006, Tunis
Tunisia
| How to cite this article: Za�em A, Charfi O, Badri T, Elaidli S. Fatal outcome of DRESS syndrome associated with esomeprazole. Indian J Dermatol Venereol Leprol 2015;81:407-408 |
Sir,
Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome is a severe, potentially life-threatening condition with a mortality rate of about 10%. This syndrome is characterized by the clinical association of fever, rash, and internal organ involvement. [1] It has been described mainly with aromatic anticonvulsants; association with proton pump inhibitors (PPIs) is extremely rare. [2],[3],[4] We report a case of DRESS syndrome associated with esomeprazole with fatal outcome.
An 84-year-old woman, with no chronic diseases and no known drug allergies complained of dyspepsia and was prescribed esomeprazole 20 mg/day. On the 8 th day after the initiation of treatment, the patient presented with fever and a rash involving the trunk. She was admitted to Habib Thameur hospital (Tunis, Tunisia). Subsequent to the admission, her general condition deteriorated. She was febrile, with a temperature of 38.9°C, and had a diffuse maculopapular rash affecting more than 50% of the skin surface [Figure - 1]. No lymphadenopathy was detected. Blood tests showed an increased white blood cells count of 22 × 10 9 cells/L with hypereosinophilia 8.2 × 10 9 cells/L. Liver enzymes were elevated: alanine aminotransferase (ALT) 265 U/L (normal ˂40 U/L), aspartate aminotransferase (AST) 196 U/L (normal ˂40 U/L), gamma glutamyl transferase (GGT) 540 U/L (normal ˂45 U/L) and serum creatinine 280 μmol/L (normal ˂115 μmol/L) with an estimated creatinine clearance of 25 mL/min. Bacteriological studies were negative. DRESS syndrome was suspected and esomeprazole stopped.
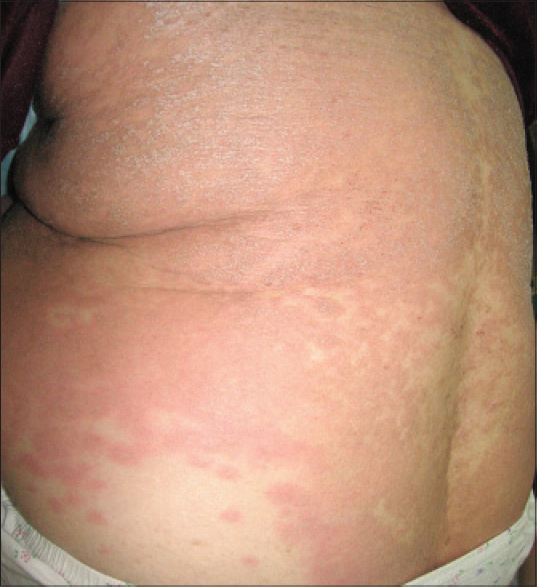 |
| Figure 1: Generalized rash on the trunk |
On the 5 th day of hospitalization, her general condition worsened with persistent fever. Intravenous methylprednisone 1 mg/kg/day was started, however she continued to worsen despite 5 days of being on systemic corticosteroids. The skin examination revealed desquamation and laboratory tests showed ALT 244 U/L, AST 203 U/L, GGT 622 U/L, and serum creatinine 433 μmol/L. The patient required intubation for respiratory support and transfer to an intensive unit care. Two days later, she died of multiple organ system failure. An autopsy was not done at the family′s request.
The diagnosis of DRESS syndrome was made in this patient based on the criteria adopted by the European group RegiSCAR, which uses a score system based on the presence of symptoms, clinical and laboratory signs. [5] In our case, the RegiScar score was 5: generalized skin rash (1), fever (0), eosinophilia (2), liver and kidney involvement (2).
Esomeprazole was suspected to be the responsible drug for the DRESS syndrome in this case based on Naranjo score. [6] This algorithm is an estimation of the probability that a drug is responsible for an adverse clinical event. It is the sum of scores of 10 items including temporal sequence, biological criteria, existence of alternative causes and effect of withdrawal and re-exposure. The total score is applied for interpretation and varies from doubtful (score of zero of less) to highly probable (score of 9 or more). The Naranjo score for esomeprazole was 4 (possible).
Proton pump inhibitors have been rarely reported as a cause for DRESS syndrome. Eight cases reported in the literature are listed in [Table - 1]; none of these cases were fatal.
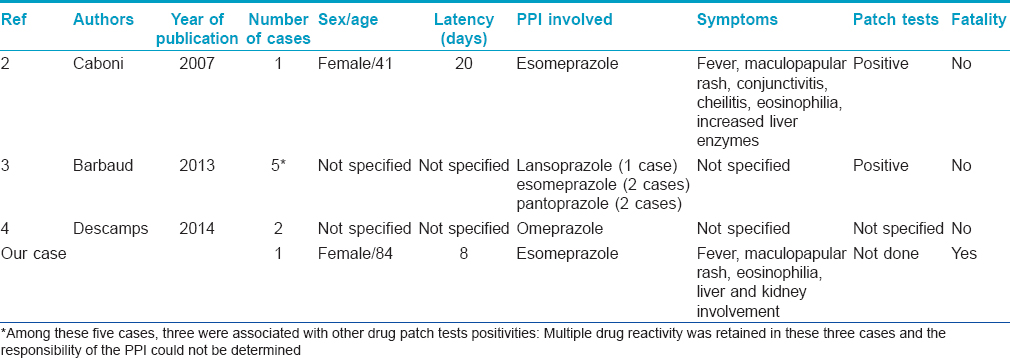
| 1. |
Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug-induced hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin Cutan Med Surg 1996;15:250-7.
[Google Scholar]
|
| 2. |
Caboni S, Gunera-Saad N, Ktiouet-Abassi S, Berard F, Nicolas JF. Esomeprazole induced DRESS syndrome. Studies of cross-reactivity among proton-pump inhibitor drugs. Allergy 2007; 62:1342-3.
[Google Scholar]
|
| 3. |
Barbaud A, Collet E, Milpied B, Assier H, Staumont D, Avenel-Audran M, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol 2013; 168:555-62.
[Google Scholar]
|
| 4. |
Descamps V, Ranger-Rogez S. DRESS syndrome. Joint Bone Spine 2014; 81:15-21.
[Google Scholar]
|
| 5. |
Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: Does a DRESS syndrome really exist? Br J Dermatol 2007; 156:609-11.
[Google Scholar]
|
| 6. |
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45.
[Google Scholar]
|
Fulltext Views
3,900
PDF downloads
2,184





