Translate this page into:
First-time versus recurrent penoscrotal extramammary Paget's disease: Clinicopathological characteristics and risk factors in 164 Chinese male patients
2 Department of Nursing, Huashan Hospital, Fudan University, Shanghai, China
Correspondence Address:
Mengbo Hu
Department of Urology, Huashan Hospital, Fudan University, No. 12 WuLuMuQi Middle Road, 200040 Shanghai
China
Haowen Jiang
Department of Urology, Huashan Hospital, Fudan University, No. 12 WuLuMuQi Middle Road, 200040 Shanghai
China
| How to cite this article: Hu J, Ge W, Mao S, Ding Q, Hu M, Jiang H. First-time versus recurrent penoscrotal extramammary Paget's disease: Clinicopathological characteristics and risk factors in 164 Chinese male patients. Indian J Dermatol Venereol Leprol 2020;86:134-140 |
Abstract
Background: Penoscrotal extramammary Paget's disease is a rare, slow-growing neoplasm with high frequency of local recurrence.
Aims: The aim of this study was to investigate the difference in clinicopathological characteristics between first-time and recurrent penoscrotal Paget's disease, and to discover the potential risk factors of recurrence.
Methods: Between January 2007 and February 2014, a total of 164 Chinese patients with biopsy-proven tramammary Paget's diseaseex in penis and scrotum underwent wide local resection in our institution. Among them, 142 patients with first-time disease and other 22 patients with recurrent disease were enrolled in this retrospective analysis.
Results: The median duration of symptoms was much shorter in recurrent disease than in first-timers (3 vs. 24 months, P < 0.001). Patients with recurrent disease tended to have lower lesion exudation rates (27.3% vs. 51.8%, P= 0.032). In addition, patients with distant stage were more likely to obtain recurrent disease compared with first-time disease (P = 0.005). Through immunohistochemical detection of extramammary Paget's specimen, we found that HER2/neu protein expression in the recurrent group was significantly higher than first-timers (P = 0.036).
Limitations: In this study, the information on familial history of most patients was insufficient. Moreover, due to the lack of follow-up data of our included cases, we were unable to evaluate the prognosis after diagnosis of extramammary Paget's disease.
Conclusion: Patients with penoscrotal Paget's disease, especially those with shorter duration of symptoms, exudation of lesions, distant-stage, Paget cells infiltrating into adnexa, and HER2/neu expression, should be followed up more carefully after surgery, as they were more likely to suffer recurrence.
Introduction
Extramammary Paget's disease is a rare cutaneous neoplasm which represents approximately 1% of all cutaneous malignant lesions.[1] The exact pathogenesis of this condition remains largely unclear. However, current evidence indicates that malignant transformation of pluripotent intraepithelial cells could lead to adenocarcinoma. Extramammary Paget's disease mainly affects elderly people of 50–80 years old on skin areas replete with full of apocrine sweat glands such as vulva, scrotum, perineum, perianal area, and axilla.[2] In male patients, penoscrotal region is the most commonly affected area.[3] The definitive diagnosis of Extramammary Paget's disease is usually delayed due to the common presenting inflammatory-like manifestations, including burning, bleeding, pruritus, and eczema, which are relatively nonspecific and generally attributed to benign diseases.[4] Most patients have a good prognosis since the spread of tumor cells is limited to the epidermis. However, once the tumor invades the dermis and subcutaneous tissue, the risks of metastasis and lethality may increase significantly. The treatment options include wide local resection with subsequent reconstruction, topical agents, immunotherapy, and radiotherapy. Unfortunately, no randomized controlled trials were performed to guide therapeutic decision-making.
At present, no definitive guidelines have been generally acknowledged for the diagnosis, treatment, and follow-up of patients with extramammary Paget's disease. Due to its rarity, previous studies mainly consisted of case reports or relatively small datasets,[5],[6],[7],[8] while studies of large population were still limited.[9],[10],[11] Our previous study showed that the wide horizontal invasion is an independent risk factor for local recurrence-free survival in patients with scrotal Paget's disease.[12] Based on the above preliminary results, the aim of this study was to further investigate the difference in clinicopathological characteristics between first-time and recurrent penoscrotal Paget's disease, thus to discover the potential risk factors of recurrence. Our results may further improve the understanding of recurrent extramammary Paget's disease and assist physicians to establish more effective diagnostic and treatment guidelines for Chinese patients.
Methods
Patients and demography
After obtaining approval of the Huashan Hospital Institutional Review Board, a retrospective study of medical records from January 2007 to February 2014 was performed to identify patients who underwent wide local resection due to penoscrotal Paget's disease. A total of 215 hospital records were screened; 33 records were excluded for incomplete data and 18 records for uncertain diagnosis. Patients were excluded if they had synchronous or metachronous internal noncutaneous malignancies, other rheumatic immune disease, or history of long-term use of immunosuppressive agents. After reviewing medical records and the patient information system, we identified a total of 164 Chinese patients with detailed admission and clinical information, as well as histopathology information at our institution. Written informed consent forms were signed by all patients prior to their inclusion in the study.
To make diagnosis, each patient received a thorough physical examination and tissue biopsy. Furthermore, medical imaging tests including chest X-ray, abdominal, and pelvic computed tomography scan were also performed to identify internal malignancies.
All 164 patients were Chinese and diagnosed with biopsy-proven Paget's disease in penis and scrotum. Among them, 142 patients with first-time penoscrotal Paget's disease and other 22 patients with recurrent disease were enrolled in this retrospective analysis.
Patient characteristics and outcome assessment
Patient characteristics were acquired mainly from electronic medical records at our institution. Basic information such as weight, height, blood pressure, medical history of hypertension and diabetes, size of lesion, and duration of symptoms were collected on admission. Body mass index (BMI) was calculated as the ratio of the patient's weight (kg) divided by the square of the patient's height (m). Induration of lesion and inguinal lymph node enlargement through physical examination were also assessed.
The evaluation of specimens obtained from wide local resection was blindly determined by two independent pathologists. Surgical margin status was evaluated by the pathologists. Malignancy infiltration was also analyzed by the presence and depth of the Paget cells' dermal invasion, which were classified as epidermis, dermis, and adnexa according to the infiltration sites. In addition, histological sections were stained with hematoxylin–eosin (H and E) and immunohistochemical staining including human epidermal growth factor receptor 2 (HER2/neu), vimentin (VIM), lymphocyte common antigen (LCA), soluble protein-100 (S-100), tumor protein 53 (P53), antigen ki67 (Ki67), human melanoma black 45 (HMB45), cytokeratin 7 (CK7), cytokeratin 20 (CK 20), and periodic acid shiff (PAS) (antibodies for above proteins were purchased from Dako, Denmark).
Primary outcomes included clinical manifestations (pruritus, erythema, exudation, rash); clinical examination results (size of lesion, delay in diagnosis, duration of symptoms, inguinal lymph node enlargement); tumor stage, being defined as local (without evidence of spread), regional (spread only to nearby nodes or tissues), or distant (spread beyond local nodes or tissues) according to the “SEER Historic Stage A” classification; histopathological assessment (positive surgical margin, malignancy infiltration); and immunohistochemical analysis (HER2/neu, VIM, LCA, S100, P53, Ki67, HMB45, CK7, CK20, and PAS).[13]
Statistical analysis
Variables were evaluated using two-tailed Student's t-test, Chi-squared analyses, and Kruskal–Wallis test where appropriate. Significance was defined as a P value ≤ 0.05. Depending on data type and statistical distribution, data were expressed as proportions or medians and interquartile range, as stated. The SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA) for Windows was used for statistical analysis.
Results
Clinical characteristics
All the 164 patients were Chinese male with complete clinical data. Among them, 142 patients (86.6%) were diagnosed with first-time penoscrotal Paget's disease, whereas other 22 patients (13.4%) with recurrent diseases.
Overall, the patients varied in age at diagnosis from 45 to 91 years (median, 70 years). The sites involved included both penis and scrotum in 54 cases, scrotum only in 73 cases, and penis only in 37 cases. Pruritus, erythema, pain, rash, erosion, and exudation were often observed in patients with extramammary Paget's disease. Of the above symptoms, pruritus (118 cases, 72%) was the most common initial symptom. In addition, the majority of patients with extramammary Paget's disease (131 cases, 80%) were initially diagnosed with eczema or inflammation, but failed to cure after the standard topical therapies. All the patients received wide surgical resection of skin lesions after the diagnosis of extramammary Paget's disease had been confirmed by biopsy [Figure - 1]. Among them, 12 patients underwent regional lymph node dissection, including nine cases of unilateral and three cases of bilateral inguinal lymph node dissection.
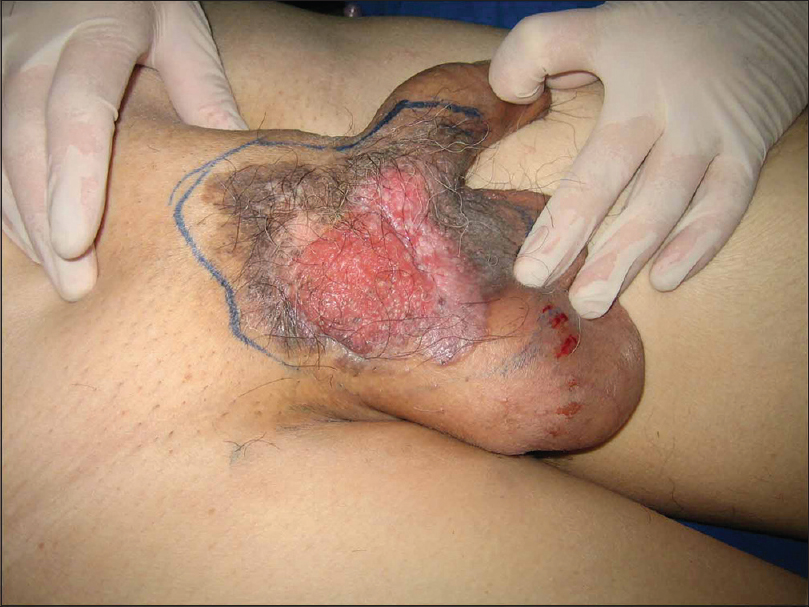 |
| Figure 1: Clinical appearance of penoscrotal Paget's disease |
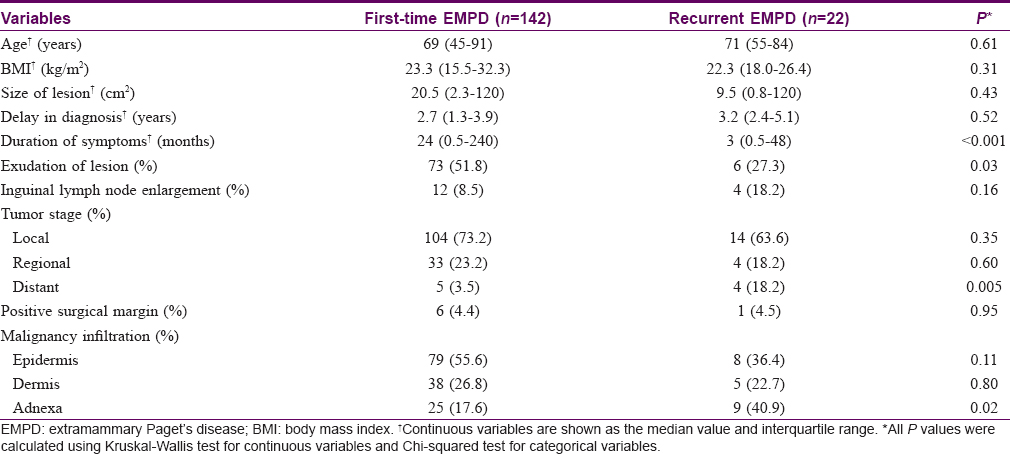
Patients were divided into two groups: first-time penoscrotal Paget's disease and recurrent disease groups, respectively. In the recurrent group, the average number of recurrences was 1.7 times per person. Clinicopathological characteristics of each group are shown in [Table - 1]. The median size of lesion was 20.5 cm2 (range, 2.3–120 cm2) in first-timers and 9.5 cm2 (range, 0.8–120 cm2) in recurrent disease. The median delay in diagnosis was 2.7 years (range, 1.3–3.9 years) in first-time disease and 3.2 years (range, 2.4–5.1 years) in recurrent disease. The median duration of symptoms was much shorter in recurrent disease than in the first-timers (3 vs. 24 months, P < 0.001). Patients with recurrent disease tended to have lower exudation rates (27.3% vs. 51.8%, P = 0.032) and potentially higher rates of inguinal lymph node enlargement (18.2% vs. 8.5%, P = 0.156). In addition, patients with distant stage were more likely to obtain recurrent disease compared with first-time disease (P = 0.005).
Histology and immunohistochemistry
Through microscopic examination, all the cases showed the prototypical pattern of in situ component. As illustrated in [Table - 1], positive surgical margins were diagnosed in 6 (4.4%) first-time extramammary Paget's disease specimens and 1 (4.5%) recurrent disease, although no significant difference was observed (P = 0.95). Paget cells are round cells usually characterized by large nuclei, prominent nucleoli, and abundant pale-staining cytoplasm, which were scattered singly or in clusters throughout the whole epidermis in H and E staining [Figure - 2]. In this study, Paget cells were exclusively detected in epidermis from 79 (55.6%) first-time disease specimens and 8 (36.4%) recurrent cases, respectively (P = 0.11). Meanwhile, Paget cells were detected in dermis from 38 (26.8%) first-time cases compared with 5 (22.7%) recurrent cases (P = 0.80). When it came to adnexa, a significantly higher proportion of Paget cells were observed in 9 (40.9%) recurrent cases compared with 25 (17.6%) first-time cases (P = 0.02). In addition, Paget cells could also be observed in the sweat glands (6.5% vs. 4.8%) and the hair follicles (3.7% vs. 2.3%).
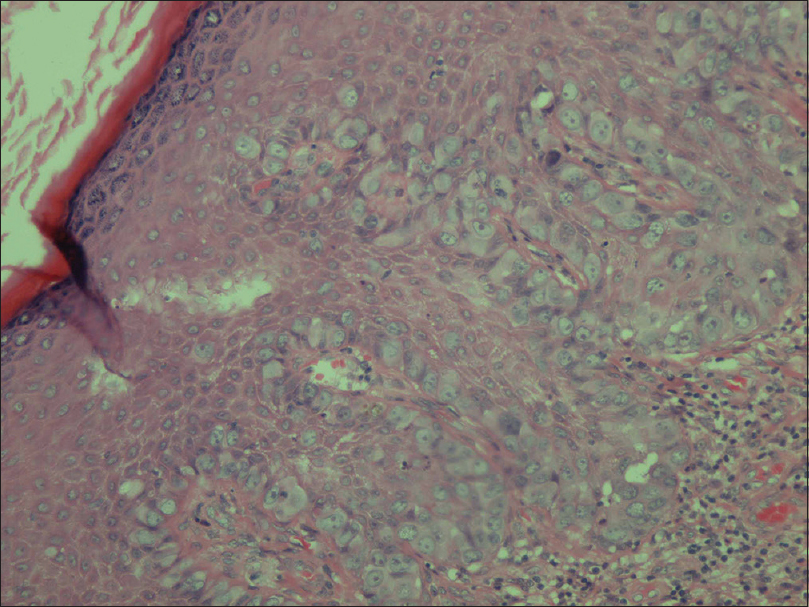 |
| Figure 2: Typical histopathological features of penoscrotal Paget's disease |
In this study, immunohistochemical detection of extramammary Paget's disease specimen was performed for various protein expression levels, including human epidermal growth factor receptor 2, vimentin, lymphocyte common antigen, soluble protein-100, tumor protein 53, antigen ki67, human melanoma black 45, cytokeratin 7, cytokeratin 20, and periodic acid shiff. In this study, we found that LCA, VIM, and HMB45 staining were all negative in both the groups. The typical expression images of other proteins are shown in [Figure - 3]. In [Table - 2], we found that HER2/neu protein expression in the recurrent group was significantly higher than the first-time group (P = 0.036), which indicated a significant correlation between HER2/neu expression and recurrent disease. Meanwhile, there were no significant differences in terms of other protein expression.
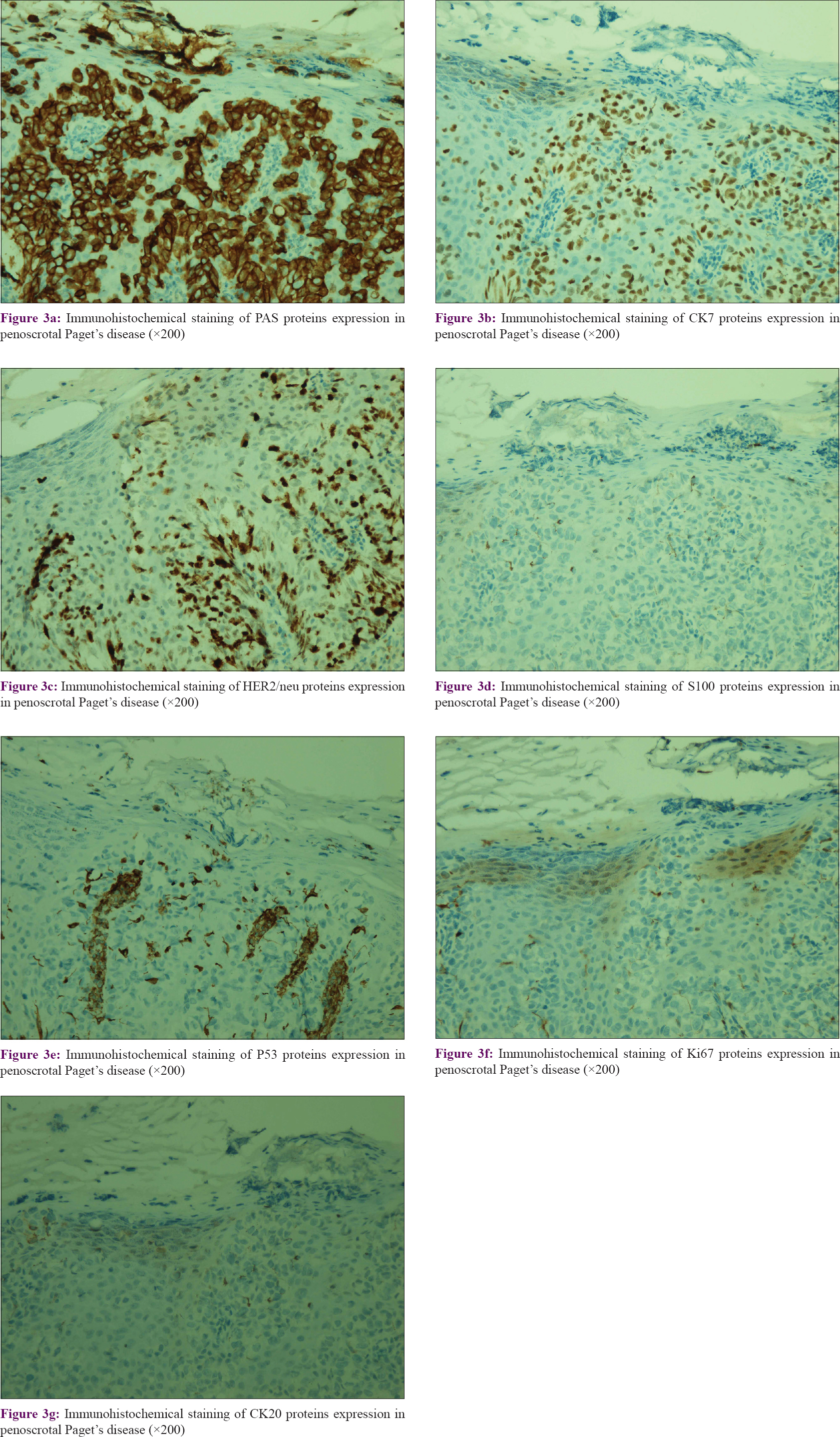 |
| Figure 3: |

Laboratory tests and associated diseases
A total of 52 patients with EMPD were detected tested for their serum prostate specific antigenlevels in this study. Among them, 7 (13.5%) patients' serum levels were above 4.0 ng/mL. As a majority of patients were elderly, associated basic disease, such as coronary heart disease (11 cases, 6.7%), diabetes mellitus (20 cases, 12.2%), hypertension (17 cases, 10.4%), or benign prostatic hyperplasia (29 cases, 17.7%), was recorded. In addition, a small proportion of patients with extramammary Paget's disease were also diagnosed with cysts through ultrasonography, including renal cysts alone (7 cases, 4.3%), hepatic cysts alone (10 cases, 6.1%), renal and hepatic cysts (9 cases, 5.5%), and testicular and epididymal cysts (8 cases, 4.9%). After reviewing the medical histories of all patients, we witnessed that seven patients (4.3%) had a history of visceral malignancies when diagnosed with extramammary Paget's disease, including stomach cancer (two cases), liver cancer (one case), colon cancer (one case), prostate cancer (two cases), and testicular cancer (one case).
Discussion
Penoscrotal Paget's disease is a rare cutaneous malignancy with small sample sizes in most published series. Our study included 164 patients with the penoscrotal EMPD in a single institution, which is mainly owing to the high-profile dermatology department at our hospital. The department of dermatology at our hospital is one of the biggest dermatology centers in China, and its annually average outpatient visits were more than 1.5 million in recent years, which has gathered a large number of patients with rare or complicated skin diseases throughout the whole country.
As seen in our patients, the median period from the awareness of initial symptoms to diagnosis was 2.7 years in first-time disease and 3.2 years in recurrent disease. Patients always gave a long history of use of ointment before diagnosis was confirmed by biopsy. In early lesions, the symptoms might be clinically confused with clinical manifestations, such as psoriasis, eczematous, and dermatitis. Over time, the lesions might become erosive, ulcerated, or even infiltrated, along with the enlargement of regional lymph nodes, which indicatesd that it might progress from in situ into invasive extramammary Paget's disease.[14] Therefore, skin biopsy is strongly recommended for patients with a progressing and protracted history of erythematous or eczematous lesions on penoscrotal area.
In this study, we identified several clinicopathological factors associated with recurrence of penoscrotal Paget's disease. Our series evaluated the factors including age, BMI, size of lesion, and delay in diagnosis from both first-time and recurrent penoscrotal Paget's groups, although no correlation was found between these factors and recurrence. Intriguingly, our data indicated that duration of symptoms, effusion, and distant stage were strongly correlated with recurrence as potential risk factors. However, some other factors including inguinal lymph node enlargement and positive surgical margin, which were initially considered to be more important, failed to be independent risk factors by data analysis. This might owe to the limited number of patients in our study. Thus, multicenter studies with larger cohorts of first-time and recurrent penoscrotal Paget's disease are still warranted to evaluate the potential risk factors for this rare entity.
Histopathological examination plays a vital role in the diagnosis of extramammary Paget's disease. In this study, we found a significantly higher proportion of Paget cells' infiltration into adnexa from patients with recurrent disease. These results also verified the previously published observations that involvement of adnexa was a common characteristic in extramammary Paget's disease, probably contributing to the development of carcinoma into deeper tissues and to the tendency for recurrence.[15],[16] Thus, infiltration of malignant cells into adnexa could be considered as another potential risk factor.
In addition, PAS staining and six immunohistochemical markers presented with different expression levels in these cases. Specifically, vimentin, lymphocyte common antigen, and human melanoma black 45 were found to be negative in, while PAS staining and CK7 showed relatively high expression levels in most tissues. Therefore, the above markers could be applied for distinguishing differential diagnosis of extramammary Paget's disease. CK7 is an marker with high sensitivity to EMPD, which could distinctly highlight Paget cells within the epidermis, sweat ducts, and pilosebaceous structures. Primary invasive extramammary Paget's disease is reported as characteristically positive for CK7 and negative for CK20, whereas secondary disease with a visceral malignancy such as colorectal or genitourinary carcinoma is often illustrated as a CK7+/CK20+ phenotype.[17],[18],[19] Furthermore, focal immunoreactivity with CK20 has been detected on primary vulvar Paget's disease in several studies.[7],[20] In contrast, HER2/neu, S100, Ki67, and P53 demonstrated various expression levels in these specimens. After evaluating the above markers, we found a significantly higher HER2/neu expression in recurrent disease group, indicating a strong correlation between HER2/neu gene and recurrence. Previous research also revealed HER2/neu overexpression in a portion of patients with vulvar Paget's, in accordance with the penoscrotal Paget's disease in this study. It is well known that HER2/neu is a remarkable biomarker for targeted gene therapy. Our results further suggested that targeted inhibition of HER2/neu might be a promising therapy for recurrent penoscrotal Paget's disease with HER2/neu expression.
In previous studies, prostate specific antigen expression was detected in Paget cells from extramammary Paget's disease cases.[21],[22] However, we found that only 7 of 52 (13.5%) patients' PSA serum levels were above the threshold (4.0 ng/mL) in this study. Furthermore, a total of seven patients (4.3%) suffered from other tumors when diagnosed with extramammary Paget's disease, which was also consistent with several published articles.[23],[24]
There were some limitations of our present study. Although with this large series, there was insufficient information on familial history for most patients with penoscrotal Paget's disease. Moreover, due to the lack of follow-up data of our included cases, we were unable to evaluate the prognosis after diagnosis of extramammary Paget's disease. Multicenter studies with larger cohorts and longer follow-up periods are needed to confirm the above-mentioned results in the future.
Conclusion
Our results suggested that shorter duration of symptoms, exudation of lesions, distant stage, Paget cells infiltrating into adnexa, and HER2/neu expression should be considered as potential risk factors of disease recurrence, which might be a better guide for the diagnosis, treatment, and follow-up of penoscrotal Paget's disease in Chinese patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
The study was financially supported by National Basic Research Program of China (2015CB943003), National Natural Science Foundation of China (81803900, 81802569, 81872102), and Shanghai Sailing Program (17YF1401700).
Conflicts of interest
There are no conflicts of interest.
| 1. |
Lam C, Funaro D. Extramammary Paget's disease: Summary of current knowledge. Dermatol Clin 2010;28:807-26.
[Google Scholar]
|
| 2. |
Kanitakis J. Mammary and extramammary Paget's disease. J Eur Acad Dermatol Venereol 2007;21:581-90.
[Google Scholar]
|
| 3. |
Moretto P, Nair VJ, Hallani SE, Malone S, Belanger E, Morash C, et al. Management of penoscrotal extramammary Paget disease: Case series and review of the literature. Curr Oncol 2013;20:e311-20.
[Google Scholar]
|
| 4. |
Kazakov DV, Spagnolo DV, Kacerovska D, Michal M. Lesions of anogenital mammary-like glands: An update. Adv Anat Pathol 2011;18:1-28.
[Google Scholar]
|
| 5. |
Ueda A, Matsumoto T, Komuro Y. Lymphangiogenesis is a predictor of nodal metastasis in extramammary Paget's disease. Histopathology 2011;58:870-4.
[Google Scholar]
|
| 6. |
Shiomi T, Yoshida Y, Shomori K, Yamamoto O, Ito H. Extramammary Paget's disease: Evaluation of the histopathological patterns of Paget cell proliferation in the epidermis. J Dermatol 2011;38:1054-7.
[Google Scholar]
|
| 7. |
Shaco-Levy R, Bean SM, Vollmer RT, Papalas JA, Bentley RC, Selim MA, et al. Paget disease of the vulva: A histologic study of 56 cases correlating pathologic features and disease course. Int J Gynecol Pathol 2010;29:69-78.
[Google Scholar]
|
| 8. |
Zhang N, Gong K, Zhang X, Yang Y, Na Y. Extramammary Paget's disease of scrotum – Report of 25 cases and literature review. Urol Oncol 2010;28:28-33.
[Google Scholar]
|
| 9. |
Kang Z, Zhang Q, Zhang Q, Li X, Hu T, Xu X, et al. Clinical and pathological characteristics of extramammary Paget's disease: Report of 246 Chinese male patients. Int J Clin Exp Pathol 2015;8:13233-40.
[Google Scholar]
|
| 10. |
Shiomi T, Noguchi T, Nakayama H, Yoshida Y, Yamamoto O, Hayashi N, et al. Clinicopathological study of invasive extramammary Paget's disease: Subgroup comparison according to invasion depth. J Eur Acad Dermatol Venereol 2013;27:589-92.
[Google Scholar]
|
| 11. |
Dai B, Kong YY, Chang K, Qu YY, Ye DW, Zhang SL, et al. Primary invasive carcinoma associated with penoscrotal extramammary Paget's disease: A clinicopathological analysis of 56 cases. BJU Int 2015;115:153-60.
[Google Scholar]
|
| 12. |
Wang L, Feng C, Zhou M, Zhou Z, Ding G, Gao P, et al. Tumor wide horizontal invasion predicts local recurrence for scrotal extramammary Paget's disease. Sci Rep 2017;7:44933.
[Google Scholar]
|
| 13. |
Herrel LA, Weiss AD, Goodman M, Johnson TV, Osunkoya AO, Delman KA, et al. Extramammary Paget's disease in males: Survival outcomes in 495 patients. Ann Surg Oncol 2015;22:1625-30.
[Google Scholar]
|
| 14. |
Shu B, Shen XX, Chen P, Fang XZ, Guo YL, Kong YY, et al. Primary invasive extramammary Paget disease on penoscrotum: A clinicopathological analysis of 41 cases. Hum Pathol 2016;47:70-7.
[Google Scholar]
|
| 15. |
Belousova IE, Kazakov DV, Michal M, Suster S. Vulvar toker cells: The long-awaited missing link: A proposal for an origin-based histogenetic classification of extramammary Paget disease. Am J Dermatopathol 2006;28:84-6.
[Google Scholar]
|
| 16. |
Shiomi T, Yoshida Y, Yamamoto O, Umekita Y. Extramammary Paget's disease: Evaluation of the adnexal status of 53 cases. Pol J Pathol 2015;66:121-4.
[Google Scholar]
|
| 17. |
Yan D, Dai H, Jin M, Zhao Y. Clinicopathologic characteristics of extramammary Paget's disease of the scrotum associated with sweat gland adenocarcinoma-a clinical retrospective study. J Chin Med Assoc 2011;74:179-82.
[Google Scholar]
|
| 18. |
Bagby CM, MacLennan GT. Extramammary Paget's disease of the penis and scrotum. J Urol 2009;182:2908-9.
[Google Scholar]
|
| 19. |
Grelck KW, Nowak MA, Doval M. Signet ring cell perianal Paget disease: Loss of MUC2 expression and loss of signet ring cell morphology associated with invasive disease. Am J Dermatopathol 2011;33:616-20.
[Google Scholar]
|
| 20. |
McCluggage WG. Recent developments in vulvovaginal pathology. Histopathology 2009;54:156-73.
[Google Scholar]
|
| 21. |
Inoguchi N, Matsumura Y, Kanazawa N, Morita K, Tachibana T, Sakurai T, et al. Expression of prostate-specific antigen and androgen receptor in extramammary Paget's disease and carcinoma. Clin Exp Dermatol 2007;32:91-4.
[Google Scholar]
|
| 22. |
Hammer A, Hager H, Steiniche T. Prostate-specific antigen-positive extramammary Paget's disease – Association with prostate cancer. APMIS 2008;116:81-8.
[Google Scholar]
|
| 23. |
Funaro D, Krasny M, Lam C, Desy D, Sauthier P, Bouffard D, et al. Extramammary Paget disease: Epidemiology and association to cancer in a Quebec-based population. J Low Genit Tract Dis 2013;17:167-74.
[Google Scholar]
|
| 24. |
Karam A, Dorigo O. Increased risk and pattern of secondary malignancies in patients with invasive extramammary Paget disease. Br J Dermatol 2014;170:661-71.
[Google Scholar]
|
Fulltext Views
4,958
PDF downloads
3,320





