Translate this page into:
Food allergen-free diet in severe atopic dermatitis related to food allergy
Correspondence Address:
Vasileios Anyfantakis
Department of Dermatology and Allergology, Poitiers University, 2 rue de la Miletrie, 86000 Poitiers
France
| How to cite this article: Marie-Helene G, Anyfantakis V, Guillet G. Food allergen-free diet in severe atopic dermatitis related to food allergy. Indian J Dermatol Venereol Leprol 2011;77:332-333 |
Sir,
The long-term management of atopic dermatitis (AD) should take into consideration environmental factors including food allergy, as it may aggravate up to 33% of the patients, [1] mainly infants and children with severe AD. [2] There is no information concerning the follow-up of patients under specific eviction of offending food. [3] The aim of our study is to evaluate the evolution of the clinical score, the applied amount of topical steroids, and IgE levels, over time, through a prospective, observational uncontrolled study including 100 children with food allergy-related, severe AD. The diagnosis of AD was based on the criteria of Hanifin and Rajka. The patients′ age ranged from one month to four years with a median age of twenty months. Clinical evaluation was based on the SCORing Atopic Dermatitis (SCORAD) score assessing the severity of erythema, edema, excoriation, dryness, lichenification and their extent, as well as pruritus and sleep, as recommended by the European task force for AD. A SCORAD score of over 40 was required in order to consider the AD to be severe. The mean consumption of topical corticosteroids was estimated according to the patients′ history. Food allergy was suspected on the basis of a detailed history and was confirmed by skin prick tests (SPT), with specific standard extracts (Stallergenes, France) or fresh food allergens. Patients with positive SPT were further evaluated for specific serum IgE through the radioallergosorbent test (RAST) or chemoluminescent assay (Dome-Hollister-Stier) techniques. Food allergy was confirmed by elimination and later by double-blind placebo-controlled food challenges, prior to initiation of the appropriate dietary intervention. Food allergy was related to egg in 67%, peanut in 54%, milk in 30%, sea shells in 26.9%, wheat flour in 16.8%, fish in 11.2%, soy in 8.9%, and mustard in 4.5%. None of these French children was vegetarian. The parents were asked to respect a specific eviction diet. The patients consulted every three months or in case of an AD flare. Informed consent was obtained for each patient and the study was approved by the Joint Committee for clinical investigation.
The mean consumption of corticosteroids was 30 g per month at the beginning of the study. The average level of total IgE was 1272 IU/ml on inclusion day and the mean SCORAD score was 53.6. Three children were excluded from the study as they did not comply with the eviction diet. In total, 97 children were observed for a protracted follow-up period of three years, which comprised the clinical score, the topical steroid consumption, and the IgE serum levels.
The effects of a specific food eviction diet on clinical improvement, topical use of corticosteroids, and total IgE, are shown in [Table - 1]. A global significant decrease over time was noticed for each of the three parameters studied. Considering the allergies diagnosed initially, reintroduction was possible within three years in 90% of the patients with allergy for egg, 95% for milk, 100% for wheat flour, and 50% for soy beans: the mean age for food allergy regression under an eviction diet was 18 months for milk, three years for flour and eggs, and none for sea food and peanuts.
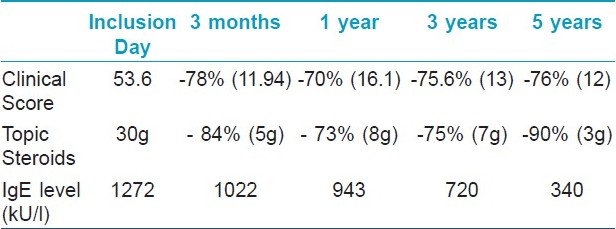
There is a considerable lack of data regarding the short- and long-term effect of an eviction diet in children with AD, as dietary exclusion remains controversial and it is assessed in unselected patients. [4] This three-year prospective study demonstrates the benefit of food eviction diet on the clinical score and topical steroids consumption, as well as on the diminution of global IgE levels. Although allergologic controls discovered the occurrence of new allergies over time, leading to the introduction of new food evictions, the clinical score remained dramatically low (average score 13 as compared to an initial value of 53.6) with an 85% reduction in the amount of applied corticosteroids and low IgE levels. At the end of the follow-up, oral challenges to patients with negative SPT and low specific IgE level permitted a reintroduction, confirming their utility as the best markers for possible allergy loss. [3] Our data confirms that a selected eviction of implicated food may lead to an important remission of AD. Previous studies have shown similar results with complete regression of symptoms in 71.4% of AD patients with food allergy. [5]
| 1. |
Burks AW, Mallory SB, Williams LW, Shirrell MA. Atopic dermatitis: Clinical relevance of food hypersensitivity reactions. J Pediatr 1988;113:447-51.
[Google Scholar]
|
| 2. |
Guillet G, Guillet MH. Natural history of sensitizations in atopic dermatitis: A 3-year follow-up in 250 children: Food allergy and high risk of respiratory symptoms. Arch Dermatol 1992;128:187-92.
[Google Scholar]
|
| 3. |
Diéguez MC, Cerecedo I, Muriel A, Zamora J, Abraira V, Camacho E, et al. Utility of diagnostic tests in the follow up of egg-allergic children. Clin Exp Allergy 2009;39:1575-84.
[Google Scholar]
|
| 4. |
Werfel T, Erdmann S, Fuchs T, Henzgen M, Kleine-Tebbe J, Lepp U, et al. Approach to suspected food allergy in atopic dermatitis. Guideline of the task force on food allergy of the german society of allergology and clinical immunology and the medical association of german allergologists and the german society of pediatric allergology. J Dtsch Dermatol Ges 2009;7:265-71.
[Google Scholar]
|
| 5. |
Resano A, Crespo E, Fernandez Benitez M, Sunz ML, Oehling A. Atopic dermatitis and food allergy. J Investig Allergol Clin Immunol 1998;8:271-6.
[Google Scholar]
|
Fulltext Views
2,368
PDF downloads
1,695





