Translate this page into:
Gaining a comprehensive understanding of pruritus
2 Department of Urology, Peking University Third Hospital, Peking, China
3 Department of Dermatology, No. 2 Hospital of Jilin University, Changchun, China
Correspondence Address:
Yaqin Zhang
No.218 Ziqiang Street, Changchun, 130041
China
| How to cite this article: Zhang H, Yang Y, Cui J, Zhang Y. Gaining a comprehensive understanding of pruritus. Indian J Dermatol Venereol Leprol 2012;78:532-544 |
Abstract
Pruritus is a common symptom associated with many dermatoses, systemic abnormalities, and psychiatric / psychosomatic diseases. Additionally, pruritus is one of the most intractable symptoms due to its complex pathogenesis involving an increasing number of mediators and receptors, undefined neurophysiologic pathways, unclear cerebral processing, and psychophysiology interaction. Clinically, the first challenge of dermatologists is how to get general and interdisciplinary vision of pruritus and to preliminarily figure it out whether there might be underlying systemic or psychosocial disorders. The second challenge is to select efficient individual tailored anti-pruritic treatment, which includes targeted drugs and cognitive-behavioral therapy.Introduction
Pruritus or itch refers to an uncomfortable sensation and emotional experience associated with an actual or perceived disturbance to the skin that provokes the desire to scratch. [1] Chronic pruritus and induced scratching behavior could have a significant impact on disease course, life quality, and healthcare costs. [2]
Though common and important, pruritus has not received much interest until the current decade, and management of pruritic conditions is still a challenge for physicians due to its multifactorial causes and complex pathophysiology. Here, we highlight a comprehensive way of understanding pruritus, including new findings on pathogenesis of pruritus, fast-emerging pruritic mediators and receptors, pathological interaction between pruritus and psychosomatic cofactors, and firstly put forward a psychosomatic cofactors-pruritus-psychiatric comorbidity cycle.
Pathogenesis of Pruritus
Pruritus is a complex process provoked by exogenous or endogenous, localized or systemic pruritogenic stimuli (chemically, electrically, mechanically, thermally, and/or psychologically). The stimuli act directly as or cause the release of peripheral or central pruritogens from various intracutaneous cell types or specific subtypes of peripheral nerve endings. After pruritogens binding with their relevant receptors, the signal generated is relayed by subsets of C fibers and second-order neurons expressing gastrin-releasing peptide receptor (GRPR) as well as spinothalamic track (STT) neurons in the spinal cord. [3] These spinal neurons form their own transmission pathway from dorsal horn to the ventrocaudal part of the nucleus medialis dorsalis (MDvc) [4] in the thalamus, which eventually project the electrochemical information (including stimulus intensity and location) to certain brain areas: Anterior cingulated cortex, insular cortex, primary and secondary somatosensory cortices, [5] and some motor areas [6] resulting in the perception of itch and an acute or chronic scratch response. Acting on any factor along the pathway of transmission of itching sensation could cause pruritus. On the other hand, psychophysiological factors could profoundly influence pruritus.
Briefly speaking, pruritus can be intracutaneous or central pathomechanisms or both. And, chronic pruritus is always associated with central mechanism and exacerbated by itch-scratch cycle.
Pruritus and pain share many similarities. Recently, it was found that GRPR is an itch-specific gene in the spinal cord. GRPR + neurons, different from the spinothalamic tract (STT) neurons (mediate both pain and itch sensation), confer the specificity of itch sensation mediated by glutamate. [7] This may elucidate how pruritogens-received by cutaneous sensory receptors, relayed by primary afferents, and discriminated and transmitted by the spinal cord-are ultimately perceived by the brain as pruritus, which could distinguish itself from pain. [8]
Mediators and Receptors
Pruritogens, also known as itch mediators, could induce an itch after being generated within or exposed to the skin. Pruritogens [9],[10],[11],[12] act peripherally, centrally or both, and their receptors have been found in various intracutaneous cell types, peripheral and central nerves, as well as sensory neurons [Table - 1]. Conventional dermatological therapies are mostly symptomatic and lack in efficacy. The fast-emerging pruritogens (e.g. interleukin-31 [13],[14] autotoxin [15] ) and receptors [(histamine H4-receptor [16] neurokinin-1 receptor (NR1-R)] serve as potential therapeutic targets for the development of imminent novel anti-pruritic drugs [e.g. interleukin-31 inhibitors, histamine H4-receptor antagonists, clonidine, [17] PI3Kγ inhibitor AS605240, [18] Cholecystokinin7S (CCK7S) and CCK8S, [19],[20],[21] aprepitant. [22] ]

Classification of Pruritus
Differentiation and an early accurate diagnosis of pruritus subtypes are imperative for revealing the underlying disorders and choosing targeted treatment. For differential diagnostic purposes, a classification of 6 categories of pruritus is proposed [23] : Category I: Dermatological diseases; Category II: Systemic diseases including diseases of pregnancy and drug-induced pruritus; Category III: Neurological; and Category IV: Psychiatric / psychosomatic diseases. Category V: Mixed overlapping and coexistence of several diseases; Category VI: Undetermined origins.
What are the features of each subtype of pruritus and how to diagnose them are the initial questions.
Category I: Pruritus from Dermatological Diseases
Pruritus is the most common accompanying symptom of many dermatological diseases, such as inflammatory diseases [e.g. atopic dermatitis (AD), psoriasis, urticaria], infectious diseases (mycotic, bacterial and viral infections, scabies, pediculosis, insect bites, folliculitis), autoimmune diseases (e.g. dermatitis herpetiformis, bullous dermatoses), neoplastic disorders (cutaneous T-cell lymphoma, cutaneous B-cell lymphoma, and leukemic infiltrates), and genodermatoses (e.g. ichthyosis vulgaris, Netherton syndrome). [23],[24]
Mechanism
Pruritus is generated in the epidermis and papillary dermis on nociceptors of unmyelinated C-fibers. [25]
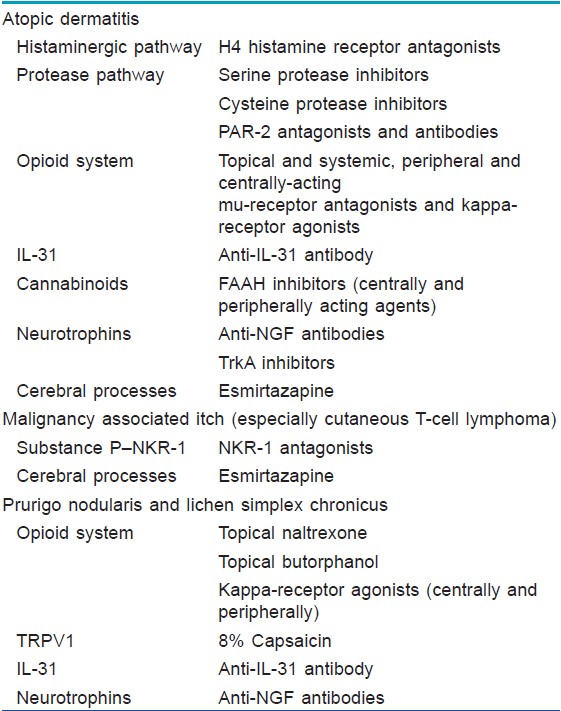
Many receptor systems are functionally expressed on these afferences. Moreover, many intracutaneous cells (keratinocytes [26] ) and immune cells (mast cells, [27] eosinophils [28] ) in the skin express a number of pruritus-associated receptors and could release inflammatory and pruritogenic substances under stimulation and finally lead to pruritus. These mechanisms are normally associated with pruritus caused by inflammatory, autoimmune, and neoplastic dermatological diseases, which are mostly characterized by chronic and severe pruritus. Recently, more mediators associated with AD, psoriasis, prurigo nodularis and lichen simplex chronicus have been elucidated [Table - 2].
Diagnosis
Diagnosis is dependent on specific skin lesions (predisposing cause , timing of onset, location, morphology, and quality). Skin examination, dermographism, microscopic examination, and skin biopsy are sometimes needed. The diagnosis of pruritus with non-specific skin lesions may be difficult. Detailed medical history, system review, and a complete physical examination aimed at detecting information and signs suggestive of underlying systemic cause should be performed. [24]
Management of pruritus from dermatological diseases
Because of long duration and a frequent combination of psychological factors and itch-scratch cycle, chronic pruritus (e.g. AD and psoriasis) caused by inflammatory, autoimmune, and neoplastic dermatological diseases is quite challenging. In order to adopt more efficacious therapy, we need to be aware of the general principles, each double-edged anti-pruritic management, and potential new targeted drugs.
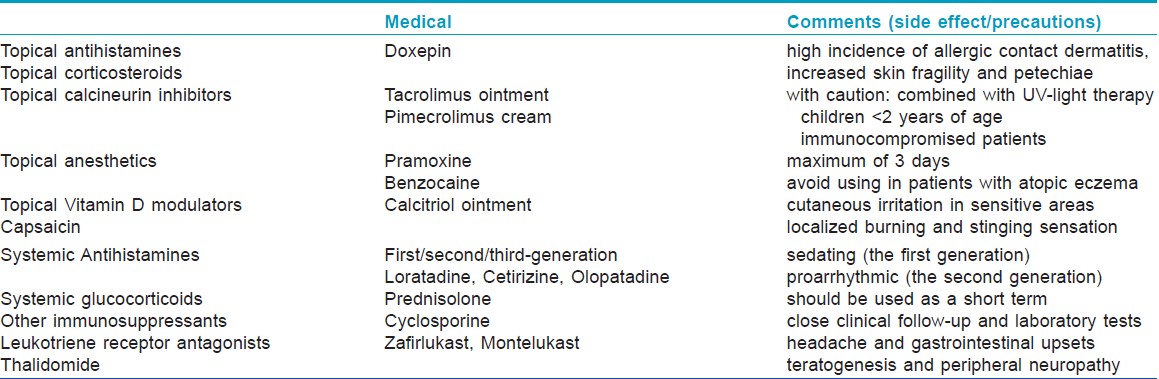
General Principles: It is experimentally and clinically demonstrated that some factors may exacerbate pruritus by lowering the threshold of an itch or impairing the skin barrier layers, and some others can alleviate pruritus by elimination of trigger factors or by improving the skin barrier function, irrespective of the underlying causes. General principles including avoiding aggravated factors and applying appropriate protective measures are essential parts of an effective treatment. [Table - 3]

Etiological Therapy: Further adoption of the etiological therapy (aimed at inhibiting the production of pruritogens or antagonizing receptors peripherally or centrally) is crucial for a long-term control of pruritus.
Present anti-pruritic therapies usually direct against a variety of targets including the epidermal barrier, immune system, or the nervous system [Table - 4]. With a better understanding of the pathological mechanisms of itch processing, identification of new medications targeting specific mediators and neuronal pathways offers a promising approach to manage pruritus more effectively. Recently, aprepitant, a NK1-R antagonist, showed rapid confirmed anti-pruritic efficacy in a small clinic experiment. [22] And, histamine H4 receptor antagonists are on their way to the clinic. Many other potential targeted drugs (e.g. anti-interleukin-31 antibody, anti-NGF antibodies, TrkA inhibitors) are under investigation and may enter the clinic in the near future.
Given that there are few specialists with sufficient expertise in chronic pruritus, and many patients have no access to advanced therapeutic options, specialized pruritus clinics are recommended. [29]
Pruritus caused by infectious diseases is commonly acute, mild or moderate, localized and is of short duration. Mechanical stimulation and/or immune cell infiltration contribute to the pathological mechanisms of pruritus. Elimination of triggering factors together with an early and specific treatment based on the primary pathogens is efficacious.
Pruritus is also a common symptom of genetic ichthyoses, which are caused by certain gene mutation leading to generation of abnormal structural proteins (keratins, filaggrin, loricrin, cornified cell envelope, etc.), deficient enzymes or transport proteins essential for the lipid metabolism in the epidermis (cholesterol sulphatase, transglutaminase, fatty acid dehydrogenase, lipoxygenases, ABCA12, etc.) [27] and finally form keratinizing disorders characterized by excessive scaling on the skin. The scale clogs the pores of the sweat glands and traps the sweat. The trapped sweat irritates the skin and leads to itchiness, redness, and occasionally small blisters. Moreover, the abnormality of certain enzymes could cause accumulation of certain molecules (e.g. leukotrienes), which can also stimulate itching. Netherton syndrome and ichthyosis vulgaris may also experience AD, and the former is a predisposition to allergies, such as asthma or food allergies. As we all know, AD and allergies always accompany pruritus. With regard to diagnose, family history and genetic counseling are important. So far, the treatment of genetic ichthyoses remains mainly symptomatic and is empirically based on the use of topical medication to ameliorate hydration, lubrication, and keratolysis, and in more severe cases, oral retinoids (vitamin A analogues), oral histamine and topical immunomodulator are also used. Recently, new drugs such as Dexeryl® and 10% N-acetylcysteine emulsion prepared in urea 5% [30] showed pleasing efficiency and good tolerance.
Category II: Pruritus from Systemic Diseases
Systemic pruritus arises from "diseases of organs" other than the skin and certain multifactorial (e.g. metabolic) states or drugs. [23] Among the diseases that can cause pruritus are renal insufficiency, cholestasis, lymphoma, polycythemia vera, solid tumors, and many others. Diseases of pregnancy and drug-induced pruritus are also divided into this group.
An underlying systemic disease is reported in 14-24% of patients who seek medical attention for pruritus. [31] And, pruritus is probably a hint to latent systemic diseases, especially when there are no signs of primary dermatological diseases. Up to 80% of patients with primary biliary cirrhosis (PBC) experience pruritus, [32] which is also the initial symptom in almost half of the patients with this disease. 15-49% of patients with chronic renal failure and up to 90% of patients receiving dialysis suffer from pruritus. [33] The prevalence of chronic pruritus has been reported as 30% in patients with Hodgkin′s disease, [34] and 15% of patients with non-Hodgkin′s disease. [35] Approximately, 30-50% patients with polycythemia vera (PV) suffer from temperature-induced itching, which may precede development of disease by several years. [36]
Mechanism
The mechanism underlying systemic pruritus is multifactorial and still poorly understood. At least 4 main hypotheses for the pathogenesis of uremic pruritus have been proposed: Dermatological abnormalities, an immune-system derangement that results in a proinflammatory state, an imbalance of the endogenous opioidergic system, and a neuropathic mechanism. [37] And, Fallahzaden et al,[38] recently found that interleukin-2 serum levels were elevated in patients with uremic pruritus.
Accumulation of bile salts, histamine, substance P, progesterone metabolites, or endogenous opioids has been controversially postulated as potential pruritogen in cholestasis in the past, but showed little convincing correlation. Most recently, lysophosphatidic acid (LPA) and autotoxin (an enzyme that produces LPA) were identified as a potent pruritogen in pruritus of cholestasis and presented better correlation with occurrence and extent of itch perception. [15],[39] Cytokines (such as interleukin-6 [40] and interleukin-8 [41] ) are found to be closely related to pathophysiology of pruritus of lymphoma.
Diagnosis
When it comes to diagnosis, a thorough history, system review, and physical examination (thyroid gland, lymph nodes, liver, and spleen) are needed. If the probability of systemic disease is low based on the above examination, a 2-week trial of symptomatic therapy (following general principles plus using conventional anti-histamine and topical corticosteroids) may be prescribed before proceeding to laboratory testing and radiological investigation. In the following 3 circumstances: Positive test, symptomatic therapy fails, or pruritus recurs, further checkups (liver / renal / thyroid panel, glucose, HIV antibody, tumor markers, and radiological investigation) are proposed. Treatment is often palliative with unsuccessful diagnosis. Here are some indications, which may increase concern for underlying systemic conditions: Old age, generalized pruritic sites, secondary skin lesions (excoriation, lichenification, prurigo nodularis) rather than primary skin lesion (e.g. wheal, papule) and itch preceding rash rather than rash before or parallel with itch. [42]
Treatment
The first step of treatment is optimizing the control of the primary diseases and following general principles. The second step is applying targeted anti-pruritic therapy (commonly neuroleptics, opioid receptor antagonists, and anti-depressant drugs). Therapeutic ladders of pruritus in end-stage renal disease (ESRD), chronic liver disease, and lymphoma are proposed. [43]
[Figure - 1] However, adverse effects of opioid receptor antagonists (gastrointestinal, cardiovascular, neurologic), and costs lead to considering these drugs as a second line approach to chronic systemic pruritus. And, they should not be used in drug addicts or in patients receiving opioid analgesics and opioid-containing medicines (cough, cold, and anti-diarrheal preparations). The most typical side effects of anti-depressant drugs are xerostomia, sleeping disorders, and sexual dysfunction. Available choices for the treatment of pruritus in pregnancy dermatoses are limited. When systemic corticosteroid treatment is necessary in pregnancy, only non-halogenated corticosteroids should be administered. [44] Ursodeoxycholic acid (UDCA) is the only treatment for intrahepatic cholestasis of pregnancy, which has been shown not only to reduce maternal pruritus but also to improve fetal prognosis. [44],[45],[46] Other bile acid exchange resins should be avoided due to the risk of causing malabsorption of vitamin K with possible consecutive bleeding complications. [47]
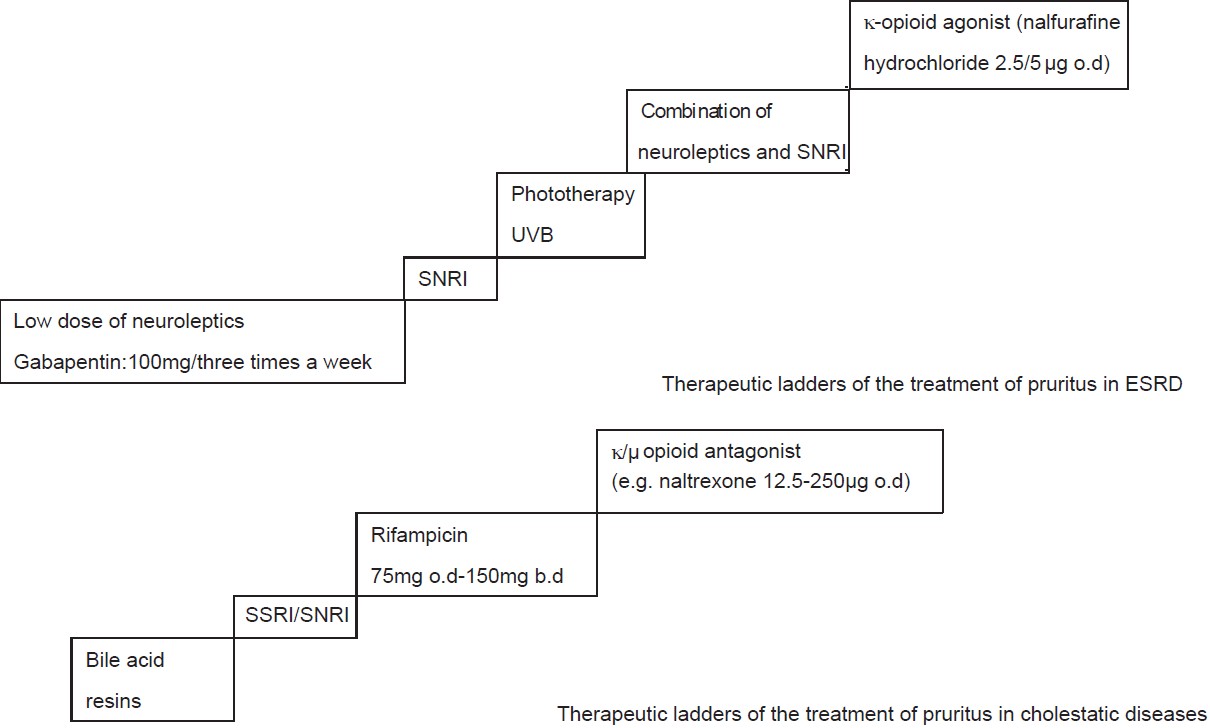 |
| Figure 1: Therapeutic ladders of the treatment of systemic pruritus |
Recently, nalfurafine hydrochloride (a novel κ opioid receptor agonist) demonstrated efficacy in a phase III, randomized, double blind and placebo controlled study in treating refractory uremic pruritus. [48] Novel imminent anti-pruritic strategies (ATX inhibitors and LPA receptor blockers for cholestatic pruritus, [19],[20],[21] aprepitant for uremic pruritus [22] ) based on specific underlying mechanisms are being developed or under evaluation and may present more efficacy and better tolerance in clinic.
Category III: Neurological Pruritus
Neurological or neuropathic pruritus terms as pruritus arising from diseases or disorders of the central or peripheral nervous system, e.g. nerve damage, nerve compression, and nerve irritation. [23] Common peripheral neuropathic pruritic diseases are postherpetic neuralgia (PHN), brachioradial pruritus (BP), Notalgia paraesthetica, keloid, and burn scars. Central neuropathic pruritic diseases include spinal tumors, Creutzfeldt-Jakob disease, [49] and multiple sclerosis. The itch sensation in most cases is chronic, persistent, and always accompanied with painful qualities (burning, stinging, biting, piercing, or tingling) and sensory damage (experienced as parasthesia, hyperesthesia, or hypothesia) in the affected areas.
Mechanisms
Mechanisms are incompletely understood. Some of the proposed mechanisms include itch associated with local nerve damage; central neuronal deprivation of afferent input, and central hypersensitivity of nerve fibers. [50]
Diagnosis
In terms of diagnosis, itch combined with pain sensation, suggestive past history and neurologic exam indicating associated sensory abnormalities may support neurological origin. Further evaluation with electromyography, nerve conduction studies, and magnetic resonance imaging is needed to locate suspected nerve impingement and to rule out space-occupying lesions.
Treatment
Therapeutic options for neuropathic itch are sparse. Neuroleptic drugs were proven to be effective in neuropathic pain, such as gabapentin and pregabalin. Side effects include sedation, neurotoxicity and/or coma in patients with reduced renal function and withdrawal symptoms with pregabalin. [51]
Botulinium toxin A injection has been reported to be successful for notalgia paresthetica and PHN neuropathic pain. [52],[53],[54] Clinical efficacy and side effects need to be assessed in further studies.
Novel drugs (Anti-NGF antibodies, TrkA inhibitors) targeting neurotrophin-induced pruritus pathway may shed new light on neurological pruritus therapy.
Category IV: Pruritus with Psychiatric / Psychosomatic Diseases
In some circumstances, chronic pruritus is indeed unrelated to skin diseases, and the skin lesions are simply secondary to scratching behavior and are highly associated with mental state.
Psychiatric pruritus is considered psychiatric in origin, which is characterized as an excessive impulse to scratch, gouge, or pick at normal skin. [50] It was found that 30% of the inpatient population with schizophrenia had pruritus with no organic basis. [55] And, patients with obsessive and compulsive disorders or eating disorders also showed high incidence of pruritus. There are actually few well-defined entities where itching is a form of psychiatric disorder (e.g. delusions of parasitosis and tactile hallucinations). Psyche-pruritus-scratch cycle is the main cause of psychiatric pruritus.
Psychological factors may perplex itch perception even in the absence of a true psychiatric morbidity. The most frequent diagnosis is "psychological impact on or psychosomatic cofactors in pruritus" (46.8%). [56]
Clinically, it has long been recognized that both acute stress (stressful life events) and chronic psychoemotional stress can precipitate or exacerbate pruritus and the scratching response. [57],[58] Gupta et al,[59] found a direct correlation between depression and pruritus severity in patients with AD, psoriasis, and chronic idiopathic urticaria (CIU). And, a close association between pruritus severity and anger in CIU was also showed. [60]
Mechanisms
The underlying mechanisms possibly involve the interaction of the skin, endocrine, nervous, and immune systems. [61] A wealth of mediators are released systemically or locally [62],[63] in response to negative emotions. Such a release has been suggested to increase sensory innervations, co-stimulate other pruritogens, perpetuate inflammation, and lower the itch threshold.
Stress activates the nervous system in a number of ways. SP-NKR1 pathway has been shown to be activated in response to stressful stimuli, both in the central and peripheral nervous system. [64] SP is an important pruritogen in the induction and maintenance of pruritus by binding to NKR1. [65],[66] And, a correlation between pruritus intensity and number of SP-positive nerves in lesional skin of psoriasis patients was observed. [67] Interestingly, the Beck′s Depression Inventory (BDI) score of the patients positively correlated with the number of SP and NKR1-positive cells in lesional skin. [66] These indicate that stress and depression may influence pruritus via substance P and NKR1. In line with these findings, it was recently reported that depressive symptomatology strongly correlated with substance P in sweat patches and plasma in patients with major depressive disorder in remission. [68] Meanwhile, stressors can activate the stress response system in the brain (hypothalamic-pituitary-adrenal axis; HPA axis) and release corticotropin-releasing hormone (CRH) into the bloodstream. Under stress, CRH is also released from dorsal root ganglia directly in the skin and could induce mast cell degranulation (releasing pruritogens: e.g. histamine, IL-31 [67] ) and subsequently potentiate pruritus signaling. [69]
Moreover, the noradrenergic system, which is highly relevant to emotional reaction, was found to play a role in itch transmission. Descending noradrenergic system exerts a tonic inhibition of itch transmission in the spinal cord mediated by α-adrenoceptors. [17],[70]
Psychosomatic cofactors -pruritus- psychiatric comorbidity cycle
Recently, a biopsychosocial model [71] of pruritus was described, which focused on the effects of personality characteristics, external stressors, cognitive disposition, behavioral and social factors, and the possible mediating role of physiological processes on pruritus.[Figure - 2] Certain personality traits (less narcissistic in psoriasis patients, dissatisfaction in urticaria patients, and need for power and social influence in AD patients [72] ), negative affectivity (e.g. depression, anxiety, neuroticism, and dissociative states), and external stressors (negative life events) are associated with the development or exacerbation of skin disorders. And, specific cognitive disposition (helplessness and worrying negatively, acceptance beneficially), behavioral (scratch and avoidant coping behavior negatively) and social factors are assumed to mediate a persons′ skin reactivity and response to pruritus.
On the other hand, patients with a pruritic disease have a high level of psychosocial (e.g. depression, suicidal ideation, public fear, sexual impairment) and occupational (absenteeism and productivity loss) morbidity. [73] Clinically, it was found that patients with prurigo nodularis and psoriasis had a high frequency of psychiatric morbidity (18% cases of anxiety and 22% cases of depression). [24],[74] Put these together, it will form a vicious psychosomatic cofactors-pruritus-psychiatric comorbidity (PPP) cycle.
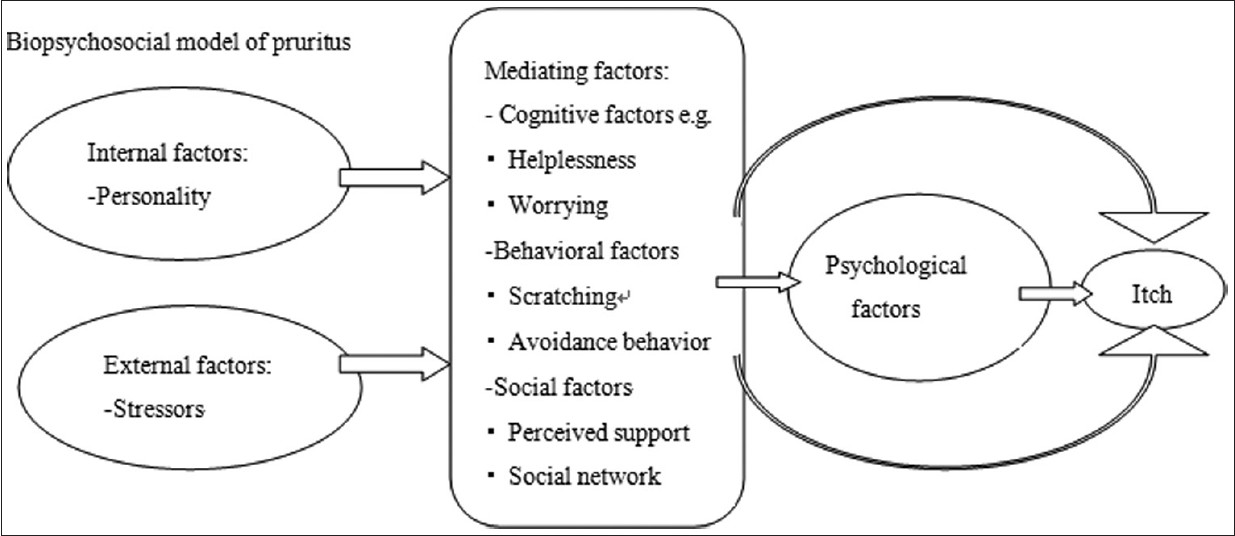 |
| Figure 2: Biopsychosocial model of Pruritus |
Heightened itch sensation, that doesn′t completely correspond to pathophysiological findings, may indicate the existence of psychosomatic cofactors. Unlike most other pruritic diseases, which cause nocturnal awakening, psychogenic pruritus rarely disturbs sleeping. And, secondary skin changes in varying stages associated can be seen, commonly on body areas that are most accessible to hands such as extensor surfaces of the arms, legs, abdomen, thighs, upper parts of the back, and shoulders with scalp and face being the most common sites. [75],[76],[77]
Diagnosis
When it comes to diagnosis of psychiatric pruritus, ruling out of systemic, neuropathic, and dermatological causes of itch should be given priority. An evaluation of whether the disease is just psychiatric, or in a combination with other pruritic diseases should also be determined. Referral to a psychiatrist or a psychologist is highly recommended after the initial evaluation by the dermatologists. [50]
In terms of psychosocial comorbidity, considering the presence of the PPP cycle, we suggest that physicians who encounter patients with pruritus should be aware of possible psychophysiological problems, and use the biopsychosocial model to evaluate the personality profiles, stressors, and the possible mediators of highly suspected patients. [78]
Treatment
Somatic treatment with anti-pruritus drugs (see the treatment of dermatological pruritus), and psychosomatic treatment including pharmacological and non-pharmacological treatment are needed.
Pharmacological treatment
Tricyclic anti-depressant (TCA), such as doxepin, amitriptyline, and trimipramine, has additional anti-histaminic effects and is of benefit in dermatological conditions such as urticaria and pruritus. And, the generally more favorable side-effect profile of the SSRIs (selective serotonin reuptake inhibitors) has made them the first-line agents in the management of psychiatric morbidity in dermatological disorders. [79] Neuroleptic medications are useful for the treatment of delusions of parasitosis, and the current drug of choice is pimizode. [80] New anti-psychotics (e.g. risperidone) are effective for psychotic syndrome and have a much safer adverse effect profile. [80]
Non-pharmacological therapy
Cognitive therapy: Besides pain, it was recently found that itch nocebo effects can be induced by only giving verbal suggestions, and a placebo effect of verbal suggestions indirectly decreased the intensity of pruritus. In the long term, these findings may facilitate the development of therapeutic strategies to reduce itch by manipulating patients′ expectations. [81]
Since catastrophizing (Inability to foresee anything other than the worst possible outcome) and helpless coping were found to be significantly related to itch, [82] patients should try to change the negative way of evaluating things.
Besides, patients and the public should be educated that chronic pruritus diseases (such as AD, psoriasis and urticaria) are not contagious, so as to reduce the social fear of the patients and to ameliorate their interpersonal relationships with their families and others.
Behavior therapy: Scratching counteracts itch (probably via segmental and suprasegmental circuits, which engage glycine and GABA-mediated inhibition of spinal itch signaling neurons [83] ). On the contrary, persistent scratching eventually damages skin, causes disfigurement, and compromises barrier function that opens the door to infection and other complications. [84]
Behavioral therapy have to be initiated in time to break the vicious circle of itching and scratching. [85]
Several techniques of relaxation, behavioral modifications, and biofeedback (a technique that involves monitoring the changes in physiologic responses to one′s thoughts or feelings) have been reported to reduce itch scratching patterns, improved their skin status, and reduce the use of dermatological care. [86]
Given that attention focus on bodily sensations is associated with higher levels of experienced itch, distracting maneuver should be taken. [87]
Combined therapy: A literature review prompted the implementation of a multidisciplinary itch coping program that comprised of a broad scope of cognitive behavioral methods (self-monitoring, guidance in skin care, and coping skills to manage itch and scratch triggering factors, stress management methods with relaxation techniques, and habit reversal). [88],[89]
And recently, an integrative program, including psychological, complementary and alternative medicine (CAM), and medical therapies was showed to be a novel, efficacious approach to decrease itch, scratching, pain, and anxiety of children suffering AD. [90]
Category V: Pruritus from Mixed Overlapping and Coexistence of Several Diseases
Patients, especially elderly patients with chronic pruritus, always have multifactorial origins, among which, metabolic, endocrine, or hematological disturbances are most common. [91],[92] In the study of Sommer et al,[93] 10.7% patients had 2, 1.9% patients had 3, 0.7% patient 4, and 0.4% patient had 5 pruritogenic pathological conditions. A high incidence of psychogenic pruritus has also been described in conjunction with pruritus due to dermatological or systemic diseases. [94] As a consequence, treatment of these patients with anti-pruritic substances exhibits several difficulties such as contraindication due to pre-existing systemic diseases, increased side effects in elderly patients, or interaction with other drugs. [95] Since the psychogenic nature of the pruritus is in most cases secondary or comorbid with severe and longstanding dermatological or systemic diseases, [96] drugs targeting primary diseases and high consideration should be given before taking psychiatric medications. And, dry skin, reduced sweating, decreased production of sebum, thicker stratum corneum, impaired skin barrier have been suggested as causes of itch in elderly people, non-specific treatment following general principles gains a significant role. [97]
Category VI: Pruritus of Undetermined Origin
Chronic pruritus with no detection of the underlying origin after completion of diagnostic tests is called PUO, which accounts for up to 45% of all cases. [93],[95],[98] Subclinical or subsided diseases are common reasons for PUO, and an association of as-yet unrelated diseases with pruritic conditions should be considered. Eisendle et al,[98] found that subclinical cholestasis was involved in the pathogenesis of PUO, and evaluation of total serum bile acid levels should be included in the diagnostic work-up of these patients. A recent study by Sonja et al,[99] showed that lactase deficiency might be an independent causal factor in the elicitation of chronic pruritus and a lactose-free diet offers a low cost, efficient, and specific therapy in patients with this kind of chronic pruritus. For older patients, the itch sensation may be due to reduced water content of the skin without overt dryness. [100],[101]
General principles should be followed before non-specific topical or oral anti-pruritus therapy (anti-histamine, corticosteroids). Opioid antagonists (naltrexone hydrochloride), and SSRIs were found to be effective treatment of severe PUO, [102],[103],[104] and gabapentin is recommended for treatment of PUO unresponsive to the usual treatment modalities. [105]
Discussion and Conclusion
Pruritus is not a diagnosis, but a symptom that associated with multiple etiologies. Clinical physicians should gain a holistic understanding of pruritus. Stepwise multi-disciplinary assessment is needed to search the underlying systemic, neural, and psychophysiological disorders. So far, effective amelioration of pruritus is still far from satisfactory. Traditional methods, such as topical treatments, UV, and systemic anti-histamine medication are symptomatic and sometimes fail to work. Novel therapeutic intervention based upon better understanding of the mediators, receptors, as well as the neurophysiological and molecular pathways is a real prospect. Although evidence-based improved treatment modalities are emerging, many of these targeted treatments appear to be effective against pruritus in animals. And, judging from the reported case number, particularly carefully-designed- controlled clinical trials are needed. The cutis and the psyche are inseparable. 106 A combination with drugs that counteract itch perception and modulate emotions is beneficial. [24] And, non-pharmacological therapy, such as psychological counseling and management of emotional or psychological problems contributing to the symptoms with cognitive-behavioral methods, is of great help, both in the short and long term.
| 1. |
Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci 2010;33:550-8.
[Google Scholar]
|
| 2. |
Ellis CN, Drake LA, Prendergast MM, Abramovits W, Boguniewicz M, Daniel CR, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol 2002;46:361- 70.
[Google Scholar]
|
| 3. |
Jeffry J, Kim S, Chen Z-F. Itch Signaling in the Nervous System. Physiology 2011;26:286-92.
[Google Scholar]
|
| 4. |
Ständer S, Raap U, Weisshaar E, Schmelz M, Mettang T, Handwerker H, et al. Pathogenesis of pruritus. J Dtsch Dermatol Ges 2011;9:456-63.
[Google Scholar]
|
| 5. |
Yosipovitch G, Ishiuji Y, Patel TS, Hicks MI, Oshiro Y, Kraft RA, et al. The brain processing of scratching. J Invest Dermatol 2008;128:1806-11.
[Google Scholar]
|
| 6. |
Schmelz M. Itch and pain. Neurosci Biobehav Rev 2010;34:171- 6.
[Google Scholar]
|
| 7. |
Koga K, Chen T, Li XY, Descalzi G, Ling J, Gu J, et al. Glutamate acts as a neurotransmitter for gastrin releasing peptide-sensitive and insensitive itch-related synaptic transmission in mammalian spinal cord. Mol Pain 2011;7:47.
[Google Scholar]
|
| 8. |
Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science 2009;325:1531-4.
[Google Scholar]
|
| 9. |
Tey HL, Yosipovitch G. Targeted treatment of pruritus: A look into the future. Br J Dermatol 2011;165:5-17.
[Google Scholar]
|
| 10. |
Tey HL, Yosipovitch G. Targeted treatment of pruritus: A look into the future. Br J Dermatol 2011;165:5-17.
[Google Scholar]
|
| 11. |
Paus R. Frontiers in pruritus research: Scratching the brain for more effective itch therapy. J Clin Invest 2006;116:1174-85.
[Google Scholar]
|
| 12. |
Tey HL, Yosipovitch G. Targeted treatment of pruritus: A look into the future. Br J Dermatol 2011;165:5-17.
[Google Scholar]
|
| 13. |
Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol 2006;117:411-7.
[Google Scholar]
|
| 14. |
Grimstad O, Sawanobori Y, Vestergaard C, Bilsborough J, Olsen UB, Gronhoj-Larsen C, et al. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: A model of atopic dermatitis. Exp Dermatol 2009;18:35-43.
[Google Scholar]
|
| 15. |
Oude Elferink RP, Kremer AE, Beuers U. Mediators of pruritus during cholestasis. Curr Opin Gastroenterol 2011;27:289-93.
[Google Scholar]
|
| 16. |
Yamaura K, Oda M, Suwa E, Suzuki M, Sato H, Ueno K. Expression of histamine H4 receptor in human epidermal tissues and attenuation of experimental pruritus using H4 receptor antagonist. J Toxicol Sci 2009;34:427-31.
[Google Scholar]
|
| 17. |
Gotoh Y, Andoh T, Kuraishi Y. Clonidine inhibits itch-related response through stimulation of α2-adrenoceptors in the spinal cord in mice. Eur J Pharmacol 2011;650:215-9.
[Google Scholar]
|
| 18. |
Pereira PJS, Lazarotto LF, Leal PC, Lopes TG, Morrone FB, Campos MM. Inhibition of phosphatidylinositol-3 kinase γ reduces pruriceptive, inflammatory, and nociceptive responses induced by trypsin in mice. Pain 2011;152:2861-9.
[Google Scholar]
|
| 19. |
Fukamachi S, Mori T, Sakabe J, Shiraishi N, Kuroda E, Kobayashi M, et al. Topical cholecystokinin depresses itch-associated scratching behavior in mice. J Invest Dermatol 2011;131:956-61.
[Google Scholar]
|
| 20. |
Pommier B, Beslot F, Simon A, Pophillat M, Matsui T, Dauge V, et al. Deletion of CCK2 Receptor in Mice Results in an Upregulation of the Endogenous Opioid System. J Neurosci 2002;22:2005-11.
[Google Scholar]
|
| 21. |
Agnes RS, Ying J, Kover KE, Lee YS, Davis P, Ma SW, et al. Structure-activity relationships of bifunctional cyclic disulfide peptides based on overlapping pharmacophores at opioid and cholecystokinin receptors. Peptides 2008;29:1413-23.
[Google Scholar]
|
| 22. |
Stander S, Siepmann D, Herrgott I, Sunderkotter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: A novel antipruritic strategy. PLoS One 2010;5:e10968.
[Google Scholar]
|
| 23. |
Ständer S, Weisshaar E, Mettang T, Szepietowski JC, Carstens E, Ikoma A, et al. Clinical Classification of Itch: A Position Paper of the International Forum for the Study of Itch. Acta Derm Venereol 2007;87:291-4.
[Google Scholar]
|
| 24. |
Cassano N, Tessari G, Vena GA, Girolomoni G. Chronic pruritus in the absence of specific skin disease: An update on pathophysiology, diagnosis, and therapy. Am J Clin Dermatol 2010;11:399-411.
[Google Scholar]
|
| 25. |
Ständer S, Weisshaar E, Luger TA. Neurophysiological and neurochemical basis of modern pruritus treatment. Exp Dermatol 2008;17:161-9.
[Google Scholar]
|
| 26. |
Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol 1998;78:84-6.
[Google Scholar]
|
| 27. |
Vahlquist A, Ganemo A, Virtanen M. Congenital ichthyosis: An overview of current and emerging therapies. Acta Derm Venereol 2008;88:4-14.
[Google Scholar]
|
| 28. |
Raap U, Goltz C, Deneka N, Bruder M, Renz H, Kapp A, et al. Brain-derived neurotrophic factor is increased in atopic dermatitis and modulates eosinophil functions compared with that seen in nonatopic subjects. J Allergy Clin Immunol 2005;115:1268-75.
[Google Scholar]
|
| 29. |
Weisshaar E, Buttner M, Ofenloch R, Matterne U. Do Patients With Chronic Pruritus Benefit From a Specialized Itch Clinic? A Patient Survey. Acta Derm Venereol 2011 [In Press].
[Google Scholar]
|
| 30. |
Bassotti A, Moreno S, Criado E. Successful Treatment with Topical N-Acetylcysteine in Urea in Five Children with Congenital Lamellar Ichthyosis. Pediat Dermatol 2011;28:451- 5.
[Google Scholar]
|
| 31. |
Reamy BV, Bunt CW, Fletcher S. A diagnostic approach to pruritus. Am Fam Physician 2011;84:195-202.
[Google Scholar]
|
| 32. |
Bergasa NV. Update on the treatment of the pruritus of cholestasis. Clin Liver Dis 2008;12:219-34, x.
[Google Scholar]
|
| 33. |
Narita I, Iguchi S, Omori K, Gejyo F. Uremic pruritus in chronic hemodialysis patients. J Nephrol 2008;21:161-5.
[Google Scholar]
|
| 34. |
Gobbi PG, Attardo-Parrinello G, Lattanzio G, Rizzo SC, Ascari E. Severe pruritus should be a B-symptom in Hodgkin's disease. Cancer 1983;51:1934-6.
[Google Scholar]
|
| 35. |
Kumar SS, Kuruvilla M, Pai GS, Dinesh M. Cutaneous manifestations of non-Hodgkin's lymphoma. Indian J Dermatol Venereol Leprol 2003;69:12-5.
[Google Scholar]
|
| 36. |
Easton P, Galbraith PR. Cimetidine treatment of pruritus in polycythemia vera. N Engl J Med 1978;299:1134.
[Google Scholar]
|
| 37. |
Aucella F, Gesuete A. Uremic pruritus: An unresolved challenge. G Ital Nefrol 2009;26:585-99.
[Google Scholar]
|
| 38. |
Fallahzadeh MK, Roozbeh J, Geramizadeh B, Namazi MR. Interleukin-2 serum levels are elevated in patients with uremic pruritus: A novel finding with practical implications. Nephrol Dial Transplant 2011;26:3338-44.
[Google Scholar]
|
| 39. |
Oude Elferink RP, Kremer AE, Martens JJ, Beuers UH. The molecular mechanism of cholestatic pruritus. Dig Dis 2011;29:66-71.
[Google Scholar]
|
| 40. |
Biggar RJ, Johansen JS, Smedby KE, Rostgaard K, Chang ET, Adami HO, et al. Serum YKL-40 and interleukin 6 levels in Hodgkin lymphoma. Clin Cancer Res 2008;14:6974-8.
[Google Scholar]
|
| 41. |
Lee HL, Eom HS, Yun T, Kim HJ, Park WS, Nam BH, et al. Serum and urine levels of interleukin-8 in patients with non-Hodgkin's lymphoma. Cytokine 2008;43:71-5.
[Google Scholar]
|
| 42. |
Koblenzer CS. Itching and the atopic skin. J Allergy Clin Immunol 1999;104:S109-13.
[Google Scholar]
|
| 43. |
Wang H, Yosipovitch G. New insights into the pathophysiology and treatment of chronic itch in patients with end-stage renal disease, chronic liver disease, and lymphoma. Int J Dermatol 2010;49:1-11.
[Google Scholar]
|
| 44. |
Ambros-Rudolph CM. Dermatoses of pregnancy - clues to diagnosis, fetal risk and therapy. Ann Dermatol 2011;23:265- 75.
[Google Scholar]
|
| 45. |
Zapata R, Sandoval L, Palma J, Hernández I, Ribalta J, Reyes H, et al. Ursodeoxycholic acid in the treatment of intrahepatic cholestasis of pregnancy. Liver Int 2005;25:548-54.
[Google Scholar]
|
| 46. |
Kondrackiene J, Beuers U, Kupcinskas L. Efficacy and safety of ursodeoxycholic acid versus cholestyramine in intrahepatic cholestasis of pregnancy. Gastroenterology 2005;129:894-901.
[Google Scholar]
|
| 47. |
Sadler LC, Lane M, North R. Severe fetal intracranial haemorrhage during treatment with cholestyramine for intrahepatic cholestasis of pregnancy. Br J Obstet Gynaecol 1995;102:169-70.
[Google Scholar]
|
| 48. |
Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: A Phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant 2010;25:1251-7.
[Google Scholar]
|
| 49. |
Shabtai H, Nisipeanu P, Chapman J, Korczyn AD. Pruritus in Creutzfeldt-Jakob disease. Neurology 1996;46:940-1.
[Google Scholar]
|
| 50. |
Yosipovitch G, Samuel LS. Neuropathic and psychogenic itch. Dermatologic Therapy 2008;21:32-41.
[Google Scholar]
|
| 51. |
Ehrchen J, Stander S. Pregabalin in the treatment of chronic pruritus. J Am Acad Dermatol 2008;58:S36-7.
[Google Scholar]
|
| 52. |
Weinfeld PK. Successful treatment of notalgia paresthetica with botulinum toxin type A. Arch Dermatol 2007;143:980-2.
[Google Scholar]
|
| 53. |
Turk U, Ilhan S, Alp R, Sur H. Botulinum toxin and intractable trigeminal neuralgia. Clin Neuropharmacol 2005;28:161-2.
[Google Scholar]
|
| 54. |
Argoff CE. A focused review on the use of botulinum toxins for neuropathic pain. Clin J Pain 2002;18:S177-81.
[Google Scholar]
|
| 55. |
Gupta MA, Gupta AK. Psychiatric and psychological co-morbidity in patients with dermatologic disorders: Epidemiology and management. Am J Clin Dermatol 2003;4:833-42.
[Google Scholar]
|
| 56. |
Weisshaar E, Dalgard F. Epidemiology of Itch: Adding to the Burden of Skin Morbidity. Acta Dermato Venereologica 2009;89:339-50.
[Google Scholar]
|
| 57. |
Gieler U, Kupfer J, Niemeier V, Brosig B. Psyche and skin: What's new? J Eur Acad Dermatol Venereol 2003;17:128-30.
[Google Scholar]
|
| 58. |
Pacan P, Grzesiak M, Reich A, Szepietowski JC. Is pruritus in depression a rare phenomenon? Acta Derm Venereol 2009;89:109-10.
[Google Scholar]
|
| 59. |
Gupta MA, Gupta AK, Schork NJ, Ellis CN. Depression modulates pruritus perception: A study of pruritus in psoriasis, atopic dermatitis, and chronic idiopathic urticaria. Psychosom Med 1994;56:36-40.
[Google Scholar]
|
| 60. |
Conrad R, Geiser F, Haidl G, Hutmacher M, Liedtke R, Wermter F. Relationship between anger and pruritus perception in patients with chronic idiopathic urticaria and psoriasis. J Eur Acad Dermatol Venereol 2008;22:1062-9.
[Google Scholar]
|
| 61. |
Arck P, Paus R. From the brain-skin connection: The neuroendocrine-immune misalliance of stress and itch. Neuroimmunomodulation 2006;13:347-56.
[Google Scholar]
|
| 62. |
Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R. Neuroimmunology of stress: Skin takes center stage. J Invest Dermatol 2006;126:1697-704.
[Google Scholar]
|
| 63. |
Paus R, Theoharides TC, Arck PC. Neuroimmunoendocrine circuitry of the 'brain-skin connection'. Trends Immunol 2006;27:32-9.
[Google Scholar]
|
| 64. |
Amatya B, El-Nour H, Holst M, Theodorsson E, Nordlind K. Expression of tachykinins and their receptors in plaque psoriasis with pruritus. Br J Dermatol 2011;164:1023-9.
[Google Scholar]
|
| 65. |
Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martin JD, et al. Tachykinins and tachykinin receptors: Structure and activity relationships. Curr Med Chem 2004;11:2045-81.
[Google Scholar]
|
| 66. |
Amatya B, El-Nour H, Holst M, Theodorsson E, Nordlind K. Expression of tachykinins and their receptors in plaque psoriasis with pruritus. Br J Dermatol 2011;164:1023-9.
[Google Scholar]
|
| 67. |
Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: Comparative evaluation of itch-associated cutaneous factors. Br J Dermatol 2003;149:718-30.
[Google Scholar]
|
| 68. |
Cizza G, Marques AH, Eskandari F, Christie IC, Torvik S, Silverman MN, et al. Elevated neuroimmune biomarkers in sweat patches and plasma of premenopausal women with major depressive disorder in remission: The POWER study. Biol Psychiatry 2008;64:907-11.
[Google Scholar]
|
| 69. |
Donelan J, Boucher W, Papadopoulou N, Lytinas M, Papaliodis D, Dobner P, et al. Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc Natl Acad Sci U S A 2006;103:7759- 64.
[Google Scholar]
|
| 70. |
Gotoh Y, Omori Y, Andoh T, Kuraishi Y. Tonic inhibition of allergic itch signaling by the descending noradrenergic system in mice. J Pharmacol Sci 2011;115:417-20.
[Google Scholar]
|
| 71. |
Verhoeven EW, de Klerk S, Kraaimaat FW, van de Kerkhof PC, de Jong EM, Evers AW. Biopsychosocial mechanisms of chronic itch in patients with skin diseases: A review. Acta Derm Venereol 2008;88:211-8.
[Google Scholar]
|
| 72. |
Bahmer JA, Kuhl J, Bahmer FA. How Do Personality Systems Interact in Patients With Psoriasis, Atopic Dermatitis and Urticaria? Acta Derm Venereol 2007;87:317-24.
[Google Scholar]
|
| 73. |
Hong J, Koo B, Koo J. The psychosocial and occupational impact of chronic skin disease. Dermatol Ther 2008;21:54-9.
[Google Scholar]
|
| 74. |
Schneider G, Hockmann J, Ständer S, Luger TA, Heuft G. Psychological factors in prurigo nodularis in comparison with psoriasis vulgaris: Results of a case-control study. Br J Dermatol 2006;154:61-6.
[Google Scholar]
|
| 75. |
Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology and approaches to treatment. CNS Drugs 2001;15:351-9.
[Google Scholar]
|
| 76. |
Koblenzer CS. Cutaneous manifestations of psychiatric disease that commonly present to the dermatologist--diagnosis and treatment. Int J Psychiatry Med 1992;22:47-63.
[Google Scholar]
|
| 77. |
Papoiu ADP, Wang H, Coghill RC, Chan YH, Yosipovitch G. Contagious itch in humans: A study of visual 'transmission' of itch in atopic dermatitis and healthy subjects. Br J Dermatol 2011;164:1299-303.
[Google Scholar]
|
| 78. |
Biro T, Ko MC, Bromm B, Wei ET, Bigliardi P, Siebenhaar F, et al. How best to fight that nasty itch - from new insights into the neuroimmunological, neuroendocrine, and neurophysiological bases of pruritus to novel therapeutic approaches. Exp Dermatol 2005;14:225-40.
[Google Scholar]
|
| 79. |
Gupta MA, Guptat AK. The use of antidepressant drugs in dermatology. J Eur Acad Dermatol Venereol 2001;15:512-8.
[Google Scholar]
|
| 80. |
Koo J, Lee CS. Delusions of parasitosis. A dermatologist's guide to diagnosis and treatment. Am J Clin Dermatol 2001;2:285-90.
[Google Scholar]
|
| 81. |
van Laarhoven AI, Vogelaar ML, Wilder-Smith OH, van Riel PL, van de Kerkhof PC, Kraaimaat FW, et al. Induction of nocebo and placebo effects on itch and pain by verbal suggestions. Pain 2011;152:1486-94.
[Google Scholar]
|
| 82. |
Kretzmer G, Gelkopf M, Melamed Y. Idiopathic pruritus in psychiatric inpatients: An explorative study. Gen Hosp Psychiatry 2008;30:344-8.
[Google Scholar]
|
| 83. |
Akiyama T, Iodi Carstens M, Carstens E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS One 2011;6:e22665.
[Google Scholar]
|
| 84. |
Sack R, Hanifin J. Scratching below the surface of sleep and itch. Sleep Med Rev 2010;14:349-50.
[Google Scholar]
|
| 85. |
Rosenbaum MS, Ayllon T. The behavioral treatment of neurodermatitis through habit-reversal. Behav Res Ther 1981;19:313-8.
[Google Scholar]
|
| 86. |
Fried RG. Nonpharmacologic treatments in psychodermatology. Dermatol Clin 2002;20:177-85.
[Google Scholar]
|
| 87. |
van Laarhoven AI, Kraaimaat FW, Wilder-Smith OH, Evers AW. Role of attentional focus on bodily sensations in sensitivity to itch and pain. Acta Derm Venereol 2010;90:46-51.
[Google Scholar]
|
| 88. |
Evers AW, Duller P, de Jong EM, Otero ME, Verhaak CM, van der Valk PG, et al. Effectiveness of a multidisciplinary itch-coping training programme in adults with atopic dermatitis. Acta Derm Venereol 2009;89:57-63.
[Google Scholar]
|
| 89. |
van Os-Medendorp H, Ros WJ, Eland-de Kok PC, Kennedy C, Thio BH, van der Schuur-van der Zande A, et al. Effectiveness of the nursing programme 'Coping with itch': A randomized controlled study in adults with chronic pruritic skin disease. Br J Dermatol 2007;156:1235-44.
[Google Scholar]
|
| 90. |
Stein TR, Sonty N, Saroyan JM. "Scratching" beneath the surface: An integrative psychosocial approach to pediatric pruritus and pain. Clin Child Psychol Psychiatry 2012;17:33- 47.
[Google Scholar]
|
| 91. |
Lober CW. Should the patient with generalized pruritus be evaluated for malignancy? J Am Acad Dermatol 1988;19:350-2.
[Google Scholar]
|
| 92. |
Kantor GR, Lookingbill DP. Generalized pruritus and systemic disease. J Am Acad Dermatol 1983;9:375-82.
[Google Scholar]
|
| 93. |
Sommer F, Hensen P, Bockenholt B, Metze D, Luger TA, Stander S. Underlying diseases and co-factors in patients with severe chronic pruritus: A 3-year retrospective study. Acta Derm Venereol 2007;87:510-6.
[Google Scholar]
|
| 94. |
Schneider G, Driesch G, Heuft G, Evers S, Luger TA, Stander S. Psychosomatic cofactors and psychiatric comorbidity in patients with chronic itch. Clin Exp Dermatol 2006;31:762-7.
[Google Scholar]
|
| 95. |
Shaw RJ, Dayal S, Good J, Bruckner AL, Joshi SV. Psychiatric medications for the treatment of pruritus. Psychosom Med 2007;69:970-8.
[Google Scholar]
|
| 96. |
Ward JR, Bernhard JD. Willan's itch and other causes of pruritus in the elderly. Int J Dermatol 2005;44:267-73.
[Google Scholar]
|
| 97. |
Weisshaar E, Dalgard F. Epidemiology of itch: Adding to the burden of skin morbidity. Acta Derm Venereol 2009;89:339-50.
[Google Scholar]
|
| 98. |
Eisendle K, Müller H, Ortner E, Talasz H, Graziadei I, Vogel W, et al. Pruritus of unknown origin and elevated total serum bile acid levels in patients without clinically apparent liver disease. J Gastroenterol Hepatol 2011;26:716-21.
[Google Scholar]
|
| 99. |
Grundmann SA, Stratmann E, Brehler R, Luger TA, Stander S. Lactase deficiency: A potential novel aetiological factor in chronic pruritus of unknown origin. Acta Derm Venereol 2011;91:698-703.
[Google Scholar]
|
| 100. |
Yesudian PD, Wilson NJ. Efficacy of gabapentin in the management of pruritus of unknown origin. Arch Dermatol 2005;141:1507-9.
[Google Scholar]
|
| 101. |
Brune A, Metze D, Luger TA, Stander S. Antipruritic therapy with the oral opioid receptor antagonist naltrexone. Open, non-placebo controlled administration in 133 patients. Hautarzt 2004;55:1130-6.
[Google Scholar]
|
| 102. |
Zylicz Z, Krajnik M, Sorge AA, Costantini M. Paroxetine in the treatment of severe non-dermatological pruritus: A randomized, controlled trial. J Pain Symptom Manage 2003;26:1105-12.
[Google Scholar]
|
| 103. |
Peer G, Kivity S, Agami O, Fireman E, Silverberg D, Blum M, et al. Randomised crossover trial of naltrexone in uraemic pruritus. Lancet 1996;348:1552-4.
[Google Scholar]
|
| 104. |
Scheinfeld N. The role of gabapentin in treating diseases with cutaneous manifestations and pain. Int J Dermatol 2003;42:491- 5.
[Google Scholar]
|
| 105. |
Chuh A, Wong W, Zawar V. The skin and the mind. Aust Fam Physician 2006;35:723-5.
[Google Scholar]
|
Fulltext Views
7,277
PDF downloads
3,384





