Translate this page into:
Genotype-phenotype spectrum and correlations in Chinese patients with keratinocytic epidermal naevus: A retrospective study of 22 cases
Corresponding author: Dr. Sheng Wang, Department of Dermatology, West China Hospital, Sichuan University, Chengdu, China. wangsheng1892@126.com
-
Received: ,
Accepted: ,
How to cite this article: Li Z, Wang S. Genotype-phenotype spectrum and correlations in Chinese patients with keratinocytic epidermal naevus: A retrospective study of 22 cases. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1292_2024
Abstract
Background
Keratinocytic epidermal naevus is characterised by hyperkeratotic lesions arranged along Blaschko’s lines. So far, multiple genes have been implicated, but there is no detailed data or genotype-phenotype correlation studies of keratinocytic epidermal naevi in Chinese patients.
Objective
To evaluate the clinical, histopathological and genetic features, genotype-phenotype correlations of keratinocytic epidermal naevus in the Chinese population.
Methods
A retrospective study of patients with keratinocytic epidermal naevi referred to the Department of Dermatology, West China Hospital, in the last four years. Medical history, clinical data, histopathological characteristics, and evidence of genetic mutations were collected from 22 unrelated Chinese patients with this problem.
Results
The distribution of the keratinocytic epidermal naevi exhibited right-side dominance. Non-epidermolytic epidermis naevus was much more common. Eight reported missense mutations were found in this study, which were detected in five genes, including HRAS, KRT10, FGFR3, GJB2, and PIK3CA. HRAS was the most commonly affected gene (9/22, 40.91%) in this study, with the c.37G>C (6/22, 27.27%) substitution representing a possible hotspot mutation. Mutation allele loads were higher in the affected lesions than blood samples. Epidermolytic epidermal naevus was found in three patients exclusively carrying KRT10 mutations. Inflammatory epidermal naevi were caused by mutations of KRT10 and PIK3CA. Most of the mosaic mutations detected in keratinocytic epidermal naevi patients were the same as germline mutations identified in systemic diseases caused by these genes.
Limitations
The retrospective nature of the study.
Conclusion
Our findings reveal the genotype-phenotype spectrum and their correlation amongst Chinese patients with keratinocytic epidermal naevi. In addition, our data underscores the importance of genetic testing in lesional skin to help characterise and categorise keratinocytic epidermal naevi, decide on a therapeutic strategy, and offer genetic counselling and prenatal diagnosis.
Keywords
Keratinocytic epidermal naevus
somatic mosaicism
deep next-generation sequencing
genotype-phenotype correlation
Chinese
Introduction
Epidermal naevus is a relatively common cutaneous mosaic disorder, typically seen at birth or developing in early childhood and evolving during puberty. Keratinocytic epidermal naevus is usually characterised clinically by gray-brown papules or plaques distributed in a linear pattern, following the lines of Blaschko and histologically by hyperplasia of epidermal keratinocytes. Clinically, keratinocytic epidermal naevus can be classified into localised or generalised variants, and inflammatory linear verrucous epidermal naevus.1 Inflammatory linear verrucous epidermal naevus could be localised or generalised. Histopathologically, keratinocytic epidermal naevus can be categorised into many distinct types, such as epidermolytic epidermal naevus, non-epidermolytic epidermal naevus, and inflammatory epidermal naevus.2 Inflammatory epidermal naevus could overlap with the other two subtypes. Herein, we describe the clinical, histopathological, and genetic features of 22 unrelated patients with keratinocytic epidermal naevi in the Chinese population.
Methods
We retrospectively reviewed the clinical, pathological, and genetic features of patients referred to our department for keratinocytic epidermal naevus between 2021 and 2024. Clinical information and medical history was collected. All patients underwent a complete physical examination at their first visit and every 12 months thereafter, in order to detect any transition towards epidermal naevus syndrome. Skin biopsies were performed for histopathological examination. Peripheral blood and lesional skin samples were obtained for the whole exome sequencing. Subsequently, targeted deep sequencing was carried out with an average coverage depth reaching × 20,000.
Results
Clinical data
The series consisted of 22 cases, 10 males and 12 females, from 22 unrelated families [Table 1]. Seventeen patients (17/22, 77.27%) were aged < 18 years while the remaining five (5/22, 22.73%) were adults. Lesions were observed in a majority of the patients since birth (21/22, 95.45%) with only one (1/22, 4.55%) developing lesions after the age of two. Unilateral distribution of the lesions was observed in most patients (17/22, 77.27%). Right-sided involvement (15/17, 88.24%) was much more common than the left-sided one (2/17, 11.76%). Fourteen patients had generalised epidermal naevus (14/22, 63.64%) while eight had the localised variant (8/22, 36.36%) [Figures 1a–1c]. All three patients with inflammatory linear verrucous epidermal naevus were female, with the lesions in all of them presenting on the right side of the body. Extracutaneous abnormalities were not documented in our patients.
| Pt N* | Sex | Age of visit | Age of onset | Family history | Clinical type | Side of the involvement | Distribution | Histopathologic type | Mutation | Reference | Mutation allele load in skin lesions (reads) | Mutation allele load in peripheral blood (reads) | Germline mutation disease |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M† | 8y° | 5mo§ | Negative | Localised | Right | Neck | Non-epidermolytic | HRAS c.37G>C; p.Gly13Arg | 4 | 13.12% (25,436/193,871) | 0 | Costello syndrome |
| 2 | F‡ | 1y | Birth | Negative | Generalised | Right | Scalp, face, trunk, groin, limbs | Non-epidermolytic | HRAS c.38G>T; p.Gly13Val | 6 | 28.13% (202,230/719,040) | 7.22% (66,506/921,430) | / |
| 3 | M | 2y | Birth | Negative | Localised | Left | Hand, chest | Non-epidermolytic | HRAS c.37G>C; p.Gly13Arg | 4 | 15.16% (30,047/198,200) | 0 | Costello syndrome |
| 4 | F | 28y | 1y | Negative | Generalised | Right | Trunk, leg | Non-epidermolytic | FGFR3 c.742C>T; p.Arg248Cys | 7 | 25.71% (13,770/53,550) | 0.86% (527/61,026) | Thanatophoric dysplasia |
| 5 | F | 4y | 1y | Negative | Generalised, ILVEN# | Right | Neck, trunk, limbs | Epidermolytic, inflammatory | KRT10 c.467G>A; p.Arg156His | 3 | 8.74% (4653/53,234) | 1.91% (1065/55,646) | EI |
| 6 | F | 10y | birth | Negative | Generalised, ILVEN | Right | Trunk, arm | Epidermolytic, inflammatory | KRT10 c.466C>T; p.Arg156Cys | 8 | 18.42% (32,005/173,715) | 2.12% (3185/150,292) | EI |
| 7 | M | 4y | 2mo | Negative | Generalised | Left and right | Scalp, face, neck, trunk, limbs | Non-epidermolytic | HRAS c.34G>A; p.Gly12Ser | 9 | 37.11% (88,706/239,059) | 2.12% (5598/263,832) | Costello syndrome |
| 8 | F | 5y | 7mo | Negative | Generalised | Left and right | Neck, trunk, limbs | Non-epidermolytic | HRAS c.34G>A; p.Gly12Ser | 9 | 32.45% (95,228/293,464) | 4.56% (10,934/239,863) | Costello syndrome |
| 9 | F | 10y | Birth | Negative | Localised | Left and right | Back, hands | Non-epidermolytic | HRAS c.37G>C; p.Gly13Arg | 4 | 17.46% (11,765/67,364) | 0 | Costello syndrome |
| 10 | M | 4y | Birth | Negative | Localised | Right | Chest | Non-epidermolytic | HRAS c.37G>C; p.Gly13Arg | 4 | 22.01% (36,256/164,697) | 0 | Costello syndrome |
| 11 | F | 4y | Birth | Negative | localised | Right | Neck | Non-epidermolytic | HRAS c.37G>C; p.Gly13Arg | 4 | 34.25% (29,269/85,461) | 0 | Costello syndrome |
| 12 | M | 7y | Birth | Negative | Generalised | Right | Scalp, trunk, limbs | Non-epidermolytic | GJB2 c.148G>A; p.Asp50Asn | 5 | 38.46% (108,640/282,464) | 1.40% (5315/380,411) | Keratitis-ichthyosis-deafness syndrome |
| 13 | F | 4y | Birth | Negative | Generalised | Left and right | Right region of trunk, right arm, lower legs | Non-epidermolytic | Not identified | / | 0 | 0 | / |
| 14 | M | 19y | 12y | Negative | Generalised | Right | Trunk, leg | Non-epidermolytic | Not identified | / | 0 | 0 | / |
| 15 | M | 13y | Birth | Negative | Generalised | Right | Trunk, groin, leg | Non-epidermolytic | Not identified | / | 0 | 0 | / |
| 16 | M | 3y | Birth | Negative | Generalised | Right | Trunk, limbs | Non-epidermolytic | Not identified | / | 0 | 0 | / |
| 17 | F | 5y | Birth | Negative | Localised | Right | Leg | Non-epidermolytic | Not identified | / | 0 | 0 | / |
| 18 | F | 47y | Birth | Negative | Localised, ILVEN | Right | Arm | Non-epidermolytic, inflammatory | PIK3CA c.1633G>A; p.Glu545Lys | 10 | 20.43% (18,657/91,309) | 0 | / |
| 19 | M | 33y | Birth | Yes (daughter suffers from EI◊) | Generalised | Left and right | Trunk, limbs | Epidermolytic | KRT10 c.467G>A; p.Arg156His | 3 | 25.08% (163,778/652,937) | 0 | EI |
| 20 | M | 13y | Birth | Negative | Localised | Left | Neck, face | Non-epidermolytic | HRAS c.37G>C; p.Gly13Arg | 4 | 27.27% (47,256/173,272) | 0 | Costello syndrome |
| 21 | F | 27y | Birth | Negative | Generalised | Right | Trunk, arm, buttock | Non-epidermolytic | FGFR3 c.742C>T; p.Arg248Cys | 7 | 28.66% (18,485/64,495) | 0 | Thanatophoric dysplasia |
| 22 | F | 6y | Birth | Negative | Generalised | Right | Neck, trunk, limbs | Non-epidermolytic | FGFR3 c.742C>T; p.Arg248Cys | 7 | 24.47% (16,468/67,304) | 0 | Thanatophoric dysplasia |
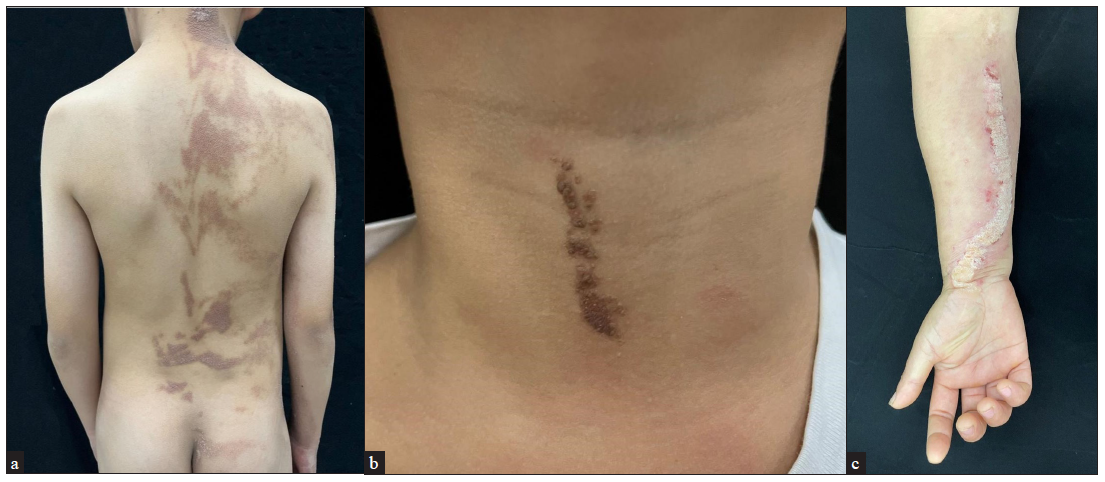
- Phenotypic spectrum of keratinocytic epidermis naevus (a) Patient 12 with generalised epidermal naevus, (b) Patient 11 with localised epidermal naevus, and (c) Patient 18 with inflammatory linear verrucous epidermal naevus.
Histopathological findings
Haematoxylin-eosin staining was performed in all the 22 patients [Table 1]. Nineteen patients were affected with non-epidermolytic epidermal naevus (19/22, 86.36%) and the remaining three had epidermolytic epidermis naevus (3/22, 13.64%). Among them, two of epidermolytic epidermal naevus (2/3, 66.67%) and one of non-epidermolytic epidermal naevus (1/3, 33.33%) overlapped with inflammatory epidermal naevus. Compared to the non-inflammatory epidermal naevus (19/22, 86.36%), inflammatory epidermal naevus (3/22, 13.64%) was rarer [Figures 2a–2c].
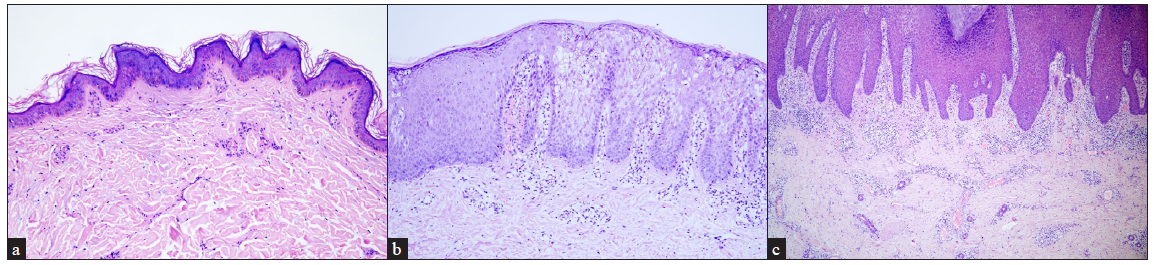
- Histopathological features of keratinocytic epidermis naevus. (a) Hyperkeratosis, verrucous acanthosis and papillomatosis in non-epidermolytic epidermal naevus (Haematoxylin and eosin, 100x), (b) Hyperkeratosis, keratinocyte vacuolisation and keratohyalin granules in epidermolytic epidermal naevus (Haematoxylin and eosin, 100x), and (c) Psoriasiform epidermal hyperplasia, orthokeratosis, parakeratosis, marked lymphocyte infiltration in the dermis in inflammatory epidermal naevus (Haematoxylin and eosin, 40x).
Genotype-phenotype correlation
Whole exome sequencing followed by targeted deep sequencing were performed using lesional skin and peripheral blood samples from all 22 patients [Table 1]. Seventeen patients (17/22, 77.27%) were found to carry mutations in five genes related to keratinocytic epidermal naevus, including HRAS, KRT10, FGFR3, GJB2, and PIK3CA. Mutations of HRAS were mostly prevalent with an incidence of 40.91% (9/22). Mutations of KRT10, FGFR3, GJB2, and PIK3CA were identified in 3/22 (13.64%), 3/22 (13.64%), 1/22 (4.55%) and 1/22 (4.55%), respectively [Figure 3a]. A total of eight missense mutations were identified in our study, which were classified as pathogenic/likely pathogenic variants in the ClinVar database and were previously reported in epidermis naevus or epidermis naevus-related syndrome.3–10 The c.37G>C substitution in HRAS represented a possible hotspot mutation (6/22, 27.27%) [Figure 3b].
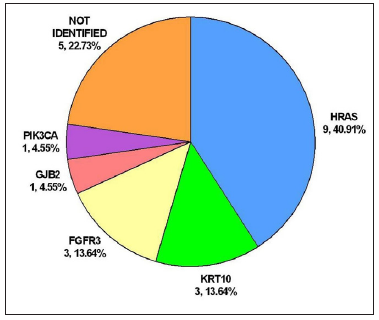
- Genotypic spectrum of keratinocytic epidermal naevus in this study. Pie chart showing the genotypic spectrum of the 22 cases studied.
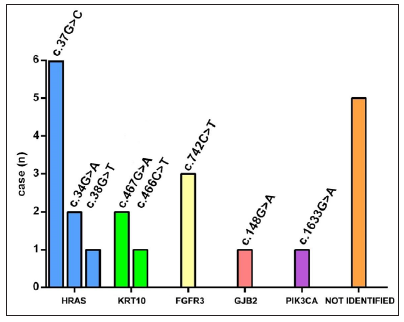
- Genotypic spectrum of keratinocytic epidermal naevus in this study. Distribution of mutation sites according to the gene involved.
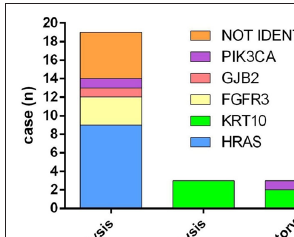
- Genotypic spectrum of keratinocytic epidermal naevus in this study. Distribution of genes according to the pathological types.
In lesional skin samples of the 17 patients with somatic mutations, the mutation allele load ranged from 8.74% to 38.46%. Only seven patients (7/22, 31.82%) were found carrying mutations in the peripheral blood, with the mutation allele load ranging from 0.86% to 7.22%. Mutations were identified in half of the blood samples in generalised epidermal naevus (7/14, 50%), while none of the mutations was observed in the blood samples in localised epidermal naevus. On the whole, in terms of mutation allele load, it was much lower in the blood (0.92 ± 1.82%) than in the lesional skin (18.95 ± 12.82%). In addition, compared to the localised epidermis naevus, the mutation allele load was significantly higher in generalised epidermal naevus, either in the peripheral blood (1.44 ± 2.13% vs. 0) or lesional skin (19.09 ± 14.48% vs. 18.71 ± 10.17%) [Table 2].
| Localised EN* (PN†, MAL‡) | Generalised EN (PN, MAL) | Total EN (PN, MAL) | p-value (Localised vs. Generalised) | |
|---|---|---|---|---|
| Total (N) | 8 | 14 | 22 | / |
| Blood | 0, 0 | 7, 1.44 ± 2.13% | 7, 0.92 ± 1.82% | 0.0730 |
| Lesional skin | 7, 18.71 ± 10.17% | 10, 19.09 ± 14.48% | 17, 18.95 ± 12.82% | 0.9492 |
| p-value (Blood vs. Lesional skin) | 0.0001 | 0.0001 | < 0.0001 | / |
In our study, 14 patients (14/22, 63.64%) had generalised epidermal naevus, seven of whom were identified with mutations in HRAS (3/14, 21.43%), KRT10 (2/14, 14.29%), FGFR3 (1/14, 7.14%) and GJB2 (1/14, 7.14%). Eight patients (8/22, 36.36%) presented with localised epidermal naevus. Mutations in HRAS (6/8, 75.00%) and PIK3CA (1/8, 12.50%) were found in seven of them. HRAS mutations occurred most frequently, including c.34G>A, c.38G>T, and c.37G>C. The c.34G>A and c.38G>T mutations were found in generalised epidermal naevus, while all the c.37G>C mutations were identified in localised epidermal naevus. Of note, acantholysis (epidermolytic epidermal naevus) was found in three patients (3/22, 13.64%) exclusively carrying KRT10 mutations. Inflammatory epidermal naevus (3/22, 13.64%) was caused by mutations in KRT10 (2/3, 66.67%) and PIK3CA (1/3, 33.33%) [Figure 3c].
Discussion
Clinically, no gender predilection was observed in the study and a majority of the patients developed keratinocytic epidermal naevus at birth. Unilateral distribution of the lesions was found in most patients, with right-sided involvement being much more common. Histopathologically, non-epidermolytic epidermal naevus was found more commonly.
Inflammatory linear verrucous epidermal naevus is a rare inflammatory epidermal naevus characterised with pruritic erythematous scaly papules and plaques occurring along Blaschko’s lines.11 In this study, three female cases of inflammatory linear verrucous epidermis naevus were included. Unlike a previous report from India12that, the left-sided inflammatory linear verrucous epidermis naevus was more common, in our study, all the the patients with this variant exhibited right-sided involvement, suggesting clinical heterogeneity in different populations. Inflammatory linear verrucous epidermal naevus was reported to be caused by mosaic mutations in GJA1, CARD14, HRAS, and KRT10.13 In our study, intriguingly, except for two patients with generalised inflammatory linear verrucous epidermal naevus caused by KRT10 mutations, a localised inflammatory linear verrucous epidermal naevus patient was found to carry a PIK3CA mutation (c.1633G>A), which has not been reported in this type previously. We speculate that compared to the PIK3CA mutation, KRT10 mutations seemingly lead to more extensive skin lesions in inflammatory linear verrucous epidermal naevus patients.
Keratinocytic epidermal naevus is a genotypically diverse mosaic disorder. Non-epidermolytic keratinocytic epidermal naevus is known to arise as a result of prenatal postzygotic mutations in either rat sarcoma/mitogen-activated protein kinase pathway (HRAS, KRAS, NRAS, BRAF) or the PI3K/AKT pathway (PIK3CA).4,14,15 Among the growth factor receptor genes found upstream of these pathways, mutations in FGFR2, FGFR3, or EGFR have also been reported to cause non-epidermolytic keratinocytic epidermal naevus.14 However, epidermolytic keratinocytic epidermal naevus is often associated with mutations in keratin genes, such as KRT1, KRT2, and KRT10.16,17 In addition, mutations in GJB2 have been found in both non-epidermolytic and epidermolytic keratinocytic epidermal naevus.5,18 Similar findings were observed in our study. Mutations of HRAS, GJB2, FGFR3 and PIK3CA were identified in non-epidermolytic keratinocytic epidermal naevus, while KRT10 was associated with epidermolytic keratinocytic epidermal naevus [Figure 3c].
Clinical manifestations of keratinocytic epidermal naevi vary according to the underlying pathogenic genes. Postzygotic mutations in HRAS and FGFR3 have been reported to cause systematized epidermal naevus, inducing skeletal abnormalities, thymoma, breast hypotrophy, acanthosis nigricans, and more.19,20 More than half (12/22, 54.55%) of the keratinocytic epidermal naevus patients carried the HRAS or FGFR3 mutations in our study. Although none of the patients in our study was found to be associated with extracutaneous symptoms, further periodical follow-up every 12 months is necessary to assess the risk of systematic involvement, especially in those who carry mutations in HRAS and FGFR3.21
HRAS was the most commonly affected gene, with c.37G>C substitution representing a hotspot mutation.4 In our study, compared to c.38G>T and c.34G>A found in the generalised epidermis naevus, c.37G>C was only identified in the localised epidermis naevus, suggesting c.37G>C mutation may lead to a milder epidermis naevus phenotype.
As a mosaic disorder, our study showed that the mutation allele load was significantly higher in generalised epidermal naevus either in the peripheral blood or lesional skin when compared to the localised variant. Meanwhile, the mutation detection rate was much lower in the blood than lesional skin in either generalised or localised epidermal naevus. Therefore, deep next-generation sequencing of the affected lesions is necessary to detect mutations in keratinocytic epidermal naevus. Genetic analysis performed solely in the peripheral blood could easily lead to false conclusions, particularly in localised epidermal naevus.22,23
It is worth noting that most mosaic mutations detected in our keratinocytic epidermal naevus patients were the same as the germline mutations identified in systemic diseases caused by these genes.11 This means that in keratinocytic epidermal naevus, an individual carrying a somatic heterozygous mutation could have co-existing gonadal mosaicism and would be at risk of transmission of the mutation to the offspring, who usually presents much more severe phenotypes.
Treatment for epidermis naevus is challenging. Recently, sirolimus which blocks mammalian target of rapamycin signalling was found effective in the patient with epidermis naevus carrying a FGFR3 mutation.24 Based on the widespread application of gene detection, targeted therapy could be a trend in the treatment of keratinocytic epidermal naevus in the future.
Limitations
This study is limited by its retrospective nature, the non-standardised documentation and small sample size.
Conclusion
To the best of our knowledge, this is the largest case series of keratinocytic epidermal naevus in the Chinese population so far. Our results reveal the genotype-phenotype spectrum and its correlation in this disease. The study underscores the importance of genetic testing in characterisation and categorisation of keratinocytic epidermal naevus, having a bearing on genetic counselling as well as prenatal diagnosis. Future therapeutic strategies could also be based on these findings.
Ethical approval
The research/study was approved by the Institutional Review Board at the ethics committee of West China Hospital, Sichuan University, number 2019-(519), dated 10 October 2019.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Picosecond 532-nm neodymium-doped yttrium aluminum garnet laser – A promising modality for the management of verrucous epidermal nevi. Lasers Med Sci. 2018;33:597-601.
- [Google Scholar]
- Adolescent onset of localized papillomatosis, lymphedema, and multiple beta-papillomavirus infection: Epidermal nevus, segmental lymphedema praecox, or verrucosis? A case report and case series of epidermal nevi. Dermatopathology (Basel). 2014;1:55-69.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidermolytic ichthyosis caused by KRT10 parental cutaneous-gonadal mosaicism: A call for prospective genetic diagnosis. Int J Dermatol. 2023;62:e583-5.
- [CrossRef] [PubMed] [Google Scholar]
- Keratinocytic epidermal nevi are associated with mosaic RAS mutations. J Med Genet. 2012;49:249-53.
- [CrossRef] [PubMed] [Google Scholar]
- Parental mosaic cutaneous-gonadal GJB2 mutation: From epidermal nevus to inherited ichthyosis-deafness syndrome. J Dermatol. 2022;49:379-82.
- [CrossRef] [PubMed] [Google Scholar]
- Expanding mutational spectrum of HRAS by a patient with Schimmelpenning-Feuerstein-Mims syndrome. J Dermatol. 2021;48:1273-6.
- [CrossRef] [PubMed] [Google Scholar]
- Widespread epidermal nevus with a postzygotic FGFR3 mutation. J Dermatol. 2017;44:e186-7.
- [CrossRef] [PubMed] [Google Scholar]
- Mutations in KRT10 in epidermolytic acanthoma. J Cutan Pathol. 2020;47:524-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Schimmelpenning-Feuerstein-Mims syndrome induced by HRAS Gly12Ser somatic mosaic mutation: Case report and literature review. J Dermatol. 2023;50:1213-5.
- [CrossRef] [PubMed] [Google Scholar]
- Somatic PIK3CA mutations in seven patients with PIK3CA-related overgrowth spectrum. Am J Med Genet A. 2017;173:978-84.
- [CrossRef] [PubMed] [Google Scholar]
- Genital/perigenital inflammatory linear verrucous epidermal nevus: A case series. Indian J Dermatol. 2015;60:592-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Adult-onset epidermal nevus with epidermolytic hyperkeratotic pattern: Case report and dermoscopic findings. Clin Case Rep. 2020;8:2398-401.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Inflammatory linear verrucous epidermal nevus (ILVEN) encompasses a spectrum of inflammatory mosaic disorders. Pediatr Dermatol. 2022;39:903-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A nonepidermolytic keratinocytic epidermal naevus associated with a postzygotic mutation in the gene encoding epidermal growth factor receptor. Br J Dermatol. 2020;182:1303-5.
- [CrossRef] [PubMed] [Google Scholar]
- Mosaic RASopathies concept: Different skin lesions, same systemic manifestations? J Med Genet. 2024;61:411-9.
- [CrossRef] [PubMed] [Google Scholar]
- Keratin 1 gene mutation detected in epidermal nevus with epidermolytic hyperkeratosis. J Invest Dermatol. 2007;127:1371-4.
- [CrossRef] [PubMed] [Google Scholar]
- First case of KRT2 epidermolytic nevus and novel clinical and genetic findings in 26 italian patients with keratinopathic ichthyoses. Int J Mol Sci. 2020;21:7707.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A somatic p.phe29del mutation of connexin 26 (GJB2) manifesting as acantholytic dyskeratotic epidermal nevus. JAMA Dermatol. 2019;155:633-5.
- [CrossRef] [PubMed] [Google Scholar]
- Postzygotic HRAS mutation causing both keratinocytic epidermal nevus and thymoma and associated with bone dysplasia and hypophosphatemia due to elevated FGF23. J Clin Endocrinol Metab.. 2014;99:E132-6.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic epidermal nevus with involvement of the oral mucosa due to FGFR3 mutation. BMC Med Genet. 2011;12:79.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidermal nevus syndromes. Handb Clin Neurol. 2015;132:291-316.
- [CrossRef] [PubMed] [Google Scholar]
- Detection and quantification of mosaic mutations in disease genes by next-generation sequencing. J Mol Diagn. 2016;18:446-53.
- [CrossRef] [PubMed] [Google Scholar]
- Disorders caused by genetic mosaicism. Dtsch Arztebl Int. 2020;116:119-25.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Topical sirolimus therapy for epidermal nevus with features of acanthosis nigricans. Pediatr Dermatol. 2019;36:554-5.
- [CrossRef] [PubMed] [Google Scholar]






