Translate this page into:
Giant syringocystadenoma papilliferum along the Blaschko’s lines
Corresponding author: Prof. Yaping Tian, Department of Dermatology and Venerology, The First Hospital of Jilin University, Changchun, Jilin Province, China. typ@jlu.edu.cn
-
Received: ,
Accepted: ,
How to cite this article: Han B, Zhang W, Li S, Wang H, Tian Y. Giant syringocystadenoma papilliferum along the Blaschko’s lines. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_659_2023
Dear Editor,
Syringocystadenoma papilliferum (SCAP) is a benign adnexal tumour originating from both apocrine and eccrine histogenesis. It was first reported as ‘naevus syringadenomatosus papilliferus’ by Stokes in 1917.1 SCAP typically presents as a papillary or solitary lesion in the head or neck region. On rare occasions, it presents as multiple small papules, occasionally along Blaschko lines. We report an unusual case of giant SCAP presenting along Blaschko lines on the thigh. We also reviewed the relevant literature on giant SCAP to further comprehend this disease.
A 24-year-old woman presented with red giant tumours over the right outer thigh in a linear configuration in 2023. The lesions began as several small papules shortly after birth, which then gradually increased in size and number without showing any other symptoms. However, the lesions progressed significantly with traumatic bleeding in 2022. The patient has no history of trauma or any notable medical issues. A physical examination revealed several red, dark-brown, moist, cauliflower-like papules measuring 1–5 mm in diameter and plaques of sizes 4 x 5 cm, 5 x 5 cm and 9 x 3 cm on the right thigh along the Blaschko lines [Figure 1]. All laboratory data were within the normal ranges. Squamous cell carcinoma, giant warts, tubular apocrine adenoma, and verrucous naevus were initially considered as differential diagnoses. Thereafter, a punch biopsy of the largest lesion revealed an exophytic configuration composed of epithelium-covered papillae [Figure 2a]. The inner layer of the papillae showed small cuboidal myoepithelial cells with hyperchromatic nuclei, while the outer layer was composed of tall columnar cells with eosinophilic cytoplasm, large vesicular nuclei and decapitation secretion [Figure 2b]. The stroma contained numerous plasma cells. Immunohistochemistry results indicated that the columnar cells were positive for AE1/AE3 [Figure 2c], CK-7 [Figure 2d] and carcinoembryonic antigen (CEA) [Figure 2e], while the cuboidal cells were positive for P63 [Figure 2f]. Plasma cells of the stroma were positive for CD38 [Figure 2g]. SCAP was diagnosed based on the clinical findings and characteristic histological features of the present case. All lesions were completely excised and closed with a tension-reducing sutures. The excised lesions were reconfirmed as SCAP by histopathological examination without any malignant transformation. The wound healed completely eight weeks after the surgery.
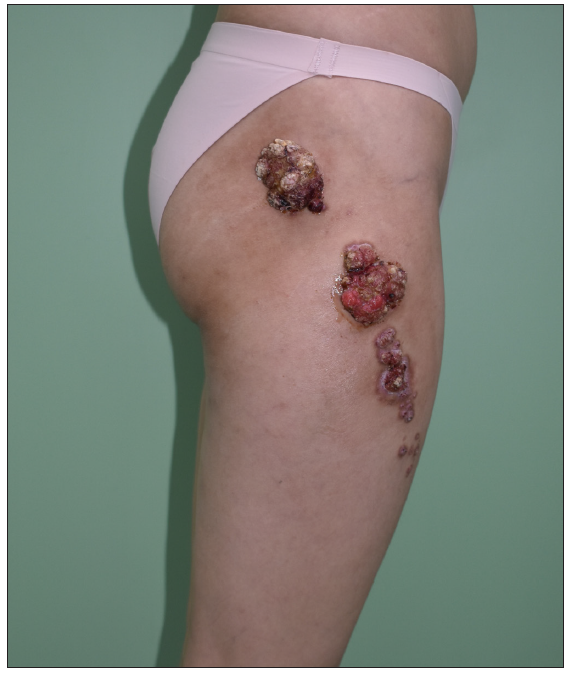
- Multiple red, dark-brown, moist, cauliflower-like papules and plaques on the right thigh along Blaschko lines.
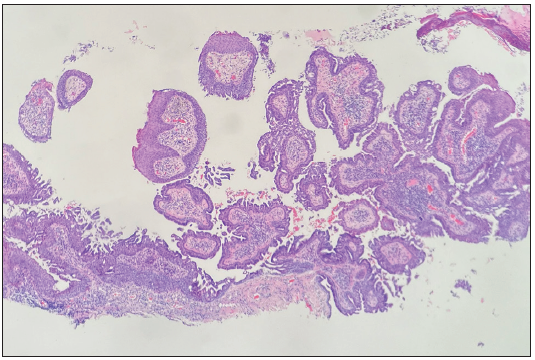
- Exophytic configuration composed of epithelium-covered papillae (Haematoxylin and Eosin, 40x).
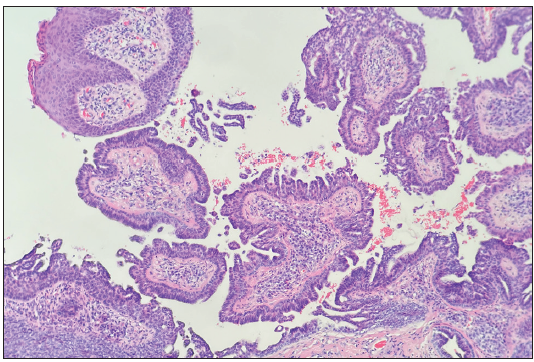
- The inner layer of the papillae included small cuboidal myoepithelial cells, while the outer layer was composed of tall columnar cells (Haematoxylin and Eosin, 100x).
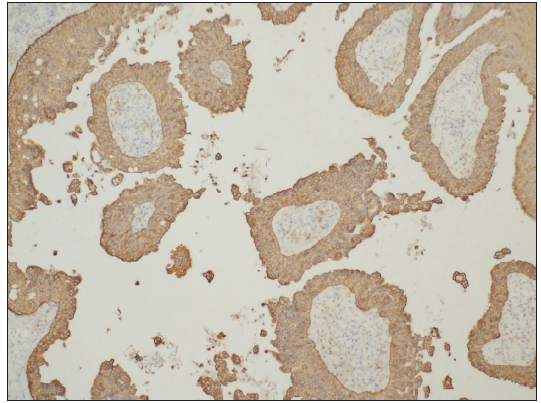
- Columnar cells were positive for CK cocktail AE1/AE3 (AE1/AE3 Immunohistochemistry, 100x).
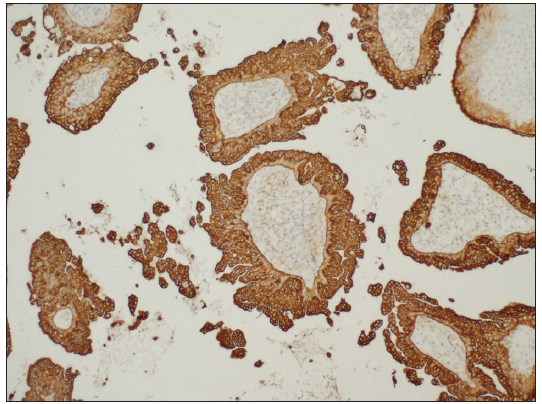
- Columnar cells were positive for CK-7 (CK-7 Immunohistochemistry, 100x).
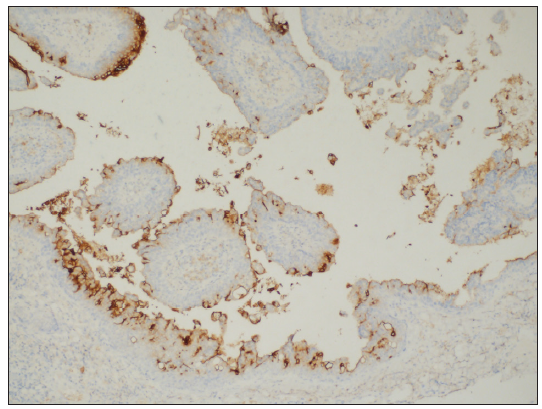
- Columnar cells were positive for CEA (CEA Immunohistochemistry, 100x).
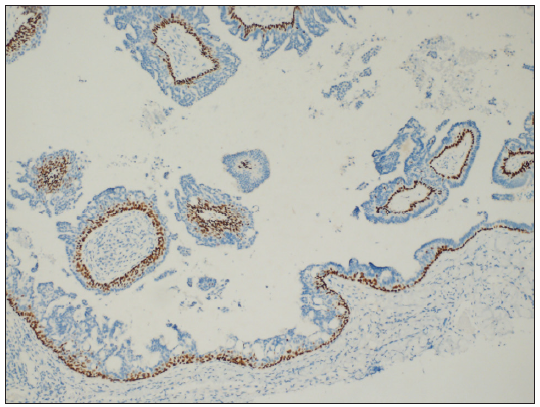
- Cuboidal cells were positive for P63 (P63 Immunohistochemistry, 100x).
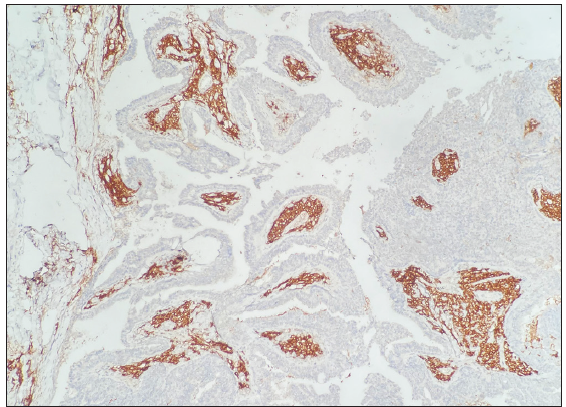
- Plasma cells of the stroma were positive for CD38 (CD38 Immunohistochemistry, 100×).
SCAP is a benign cutaneous neuroma derived from both apocrine and eccrine histogenesis and composed of undifferentiated pluripotential cells.2 Clinically, it typically presents as a dark-brown or grey papillary or rather warty, sometimes crusty, excrescence with a moist appearance that may manifest at birth or develop during childhood.2 Head and neck regions are most commonly involved sites.2 The following three clinical types of SCAP have been described so far: solitary nodular type, plaque type and linear type, of which, the linear type is rare.3 So far, there have been only four published cases of giant SCAP reported in the English literature,1,4–6 and none has been described to have presented on the thigh as a linear type. Based on the literature review, the age at the time of diagnosis, sex, the age of onset, location, appearance, clinical features, and any accompanying diseases are summarised in Table 1. Histologically, SCAP has a characteristic that makes it readily recognisable owing to its papillary projection consisting of two rows of cells – columnar and cuboidal, and the stroma contains numerous plasma cells.2 SCAP has been reported to be positive for carcinoembryonic antigen, cytokeratin AE1 (CK AE1), and epithelial membrane antigen (EMA) in columnar cells and positive for P63 in cuboidal cells.2 The clinical and histological features of the present case were found to be consistent with those of SCAP.
| Author (date) | Age (year)/sex | Age of onset (year) | Location | Appearance | Clinical features | Accompanying diseases |
|---|---|---|---|---|---|---|
| Kar et al.1 (2012) | 12/M | Since birth | Lower back | A 20-cm long and 7-cm wide fleshy cauliflower-like erythematous papulonodular lesion arranged in a linear array | No | No |
| Yaghoobi et al.4 (2009) | 20/F | 19 | Left-sided occipital part of the scalp | A linear lesion, highly elevated, moist, fetid, vegetated and pinkish, measuring 8 × 3 × 2 cm in diameter | No | Hypochromic, microcytic anaemia |
| Sardesai et al.5 (2009) | 30/F | 25 | The medial aspect of the right thigh | Two skin lesions: (large) 6 × 7 cm and (small) 1 × 3 cm | No | Condyloma acuminata |
| Kaehler et al.6 (2005) | 43/M | Since birth | The left side of the ventral thorax | A giant, 4 × 6 cm brownish, verrucous tumour with several scars | No | No |
| Our case (2023) | 24/F | Since birth | Right thigh along Blaschko line | Several red, dark-brown, moist, cauliflower-like papules, measuring 1–5 mm in diameter, and plaques of size 4 × 5 cm, 5 × 5 cm, and 9 × 3 cm, respectively, on the right thigh along the Blaschko lines | Traumatic bleeding | No |
SCAP has recently been reported to be associated with somatic activating mutations in the HRAS, KRAS or BRAF oncogenes.7 Owing to financial reasons, the patient refused genetic testing. Surgical excision is the first-line treatment option for SCAP and CO2 laser may be considered for anatomic sites unfavourable to surgical excision.1 All lesions of the present patient were removed surgically.
In summary, we report a case of Blaschko-linear SCAP located on the thigh with a giant appearance. Our case findings highlight the important role played by histopathological examination to rule out other cutaneous tumours demonstrating similar characteristics.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Giant linear syringocystadenoma papilliferum of the back. Indian J Dermatol Venereol Leprol. 2012;78:123.
- [CrossRef] [PubMed] [Google Scholar]
- Pathology of the skin with clinical correlations. Elsevier Limited; 2012. p. :1510-11.
- Syringocystadenoma papilliferum in the scalp, with a linear presentation. An Bras Dermatol. 2023;98:406-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Giant linear syringocystadenoma papilliferum on scalp. Indian J Dermatol Venereol Leprol. 2009;75:318-19.
- [CrossRef] [PubMed] [Google Scholar]
- Giant condyloma acuminata with syringocystadenoma papilliferum. Indian J Dermatol Venereol Leprol. 2009;75:330.
- [CrossRef] [PubMed] [Google Scholar]
- Giant naevoid syringocystadenoma papilliferum. Acta Derm Venereol. 2005;85:453-4.
- [CrossRef] [PubMed] [Google Scholar]
- Activating mutations in the RAS/mitogen-activated protein kinase signaling pathway in sporadic trichoblastoma and syringocystadenoma papilliferum. Hum Pathol. 2015;46:272-6.
- [CrossRef] [PubMed] [Google Scholar]





