Translate this page into:
Herpes simplex virus reactivation in patients of COVID-19-associated mucormycosis
Corresponding author: Dr. Anupama Bains, Department of Dermatology, Venereology and Leprology, AIIMS Jodhpur, India, whiteangel2387@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bains A, Kour N, Madhudiya R. Herpes simplex virus reactivation in patients of COVID-19-associated mucormycosis. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_281_2024
Dear Editor,
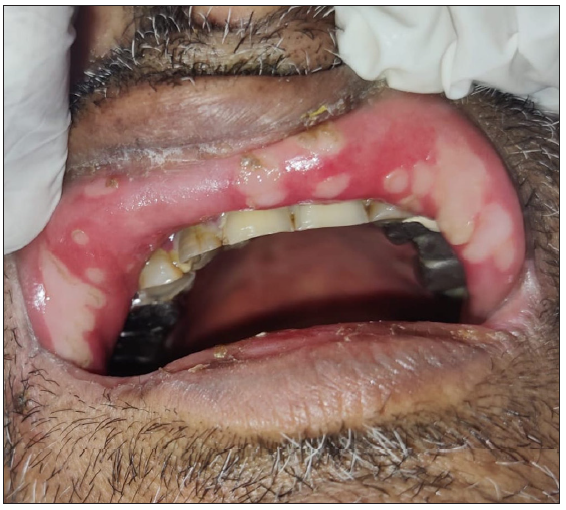
An unprecedented epidemic of mucormycosis was noted especially during the second wave of COVID-19.1 In this period between May and June 2021, we received referrals from the otorhinolaryngology department for oral lesions in patients of COVID-19-associated mucormycosis. This prompted us to collect information about these patients prospectively. A total of eight cases were observed. The age of patients ranged from 33 to 55 years with a mean age of 43.75 years. There were six males and two females. All patients were confirmed cases of rhinocerebral mucormycosis diagnosed by computed tomography scan/magnetic resonance imaging studies and mycology findings. All of these patients had COVID-19 infection diagnosed six to seven weeks before the onset of mucormycosis by real-time reverse transcription – polymerase chain (RT-PCR) on nasopharyngeal swabs. Half of the patients had associated type II diabetes mellitus whereas no associated risk factor was observed in others. A dermatology referral was sought for oral ulcers in these patients. The duration of oral lesions ranged from three to six days with the average duration being 4.4 days. The most common sites involved were the oral cavity (four patients, 50%), face (three patients, 37.5%), and both the oral cavity and face (one patient 12.5%). Lesions were painful in all the patients. The size of lesions ranged from 0.1 mm to 5 cm. In the oral cavity, the tongue was the most commonly affected site followed by the lips and the angle of the mouth [Table 1]. Most common morphology of oral lesions noted was large geographic ulcer (5) followed by ulcers with polycyclic margins (3), round to oval ulcers (2) and yellowish plaque with cobblestone appearance (1). Patients with involvement of the face had mostly monomorphic crusted erosions (four patients) with sizes ranging from 1 to 3 mm [Figures 1a to 1f]. There was no difference in the morphology or severity of lesions in diabetic and non-diabetic patients. There were no lesions elsewhere in the body as well as no history of similar lesions in the past. Potassium hydroxide smear examination from lesions were negative for fungus in all the cases. Tzanck smear from lesions showed multinucleate giant cells in four patients, was negative in three patients, and not done in one patient. Patients were diagnosed with herpetic stomatitis and facial herpes and were started on intravenous or oral acyclovir depending on the severity of the disease.
| Patient | Duration of herpetic lesions | Site of herpetic lesions | Morphology of herpetic lesions | Size of herpetic lesions | Symptoms | Tzanck smear | Treatment given |
|---|---|---|---|---|---|---|---|
| 1. | 4 days | Oral cavity | Multiple ulcers on the upper lip, lower lip, and tongue with polycyclic margins | 1–5 cm | Painful | Multinucleate giant cells | Intravenous acyclovir 400 mg TDS |
| 2. | 3 days | Oral cavity | Multiple yellowish plaques giving a cobblestone appearance along with round to oval ulcers on the dorsal aspect of the tongue | 0.2–4 cm | Painful | Multinucleate giant cells | Intravenous acyclovir |
| 3. | 6 days | Oral cavity | On the dorsal aspect of the tongue; a large geographic ulcer covered with purulent slough | 1–5 cm | Painful | Multinucleate giant cells | Intravenous acyclovir |
| 4. | 5 days | Oral cavity | Multiple ulcers round to oval, some having polycyclic margins on the tongue | 0.5–5 cm | Painful | Negative | Intravenous acyclovir |
| 5. | 3 days | Face | Monomorphic crusted erosions on nose, right cheek, near nostril | 2–3 mm | Painful | Multinucleate giant cells | Oral acyclovir |
| 6. | 4 days | Face | Monomorphic crusted erosions | 2–3 mm | Painful | Negative | Oral acyclovir |
| 7. | 5 days | Face and oral cavity | Multiple well-defined ulcers with polycyclic margins and purulent base in tongue, angle of mouth. on chin, cheeks, and nose, circular to oval shaped tiny crusted erosions | 1–3 mm on the face and 1–5 cm in the oral cavity | Painful | Negative | Intravenous acyclovir |
| 8. | 5 days | Face | Monomorphic round-shaped crusted erosions on the nose and near the right nostril | 2–3 mm | Painful | Not done | Oral acyclovir |
TDS: three times a day
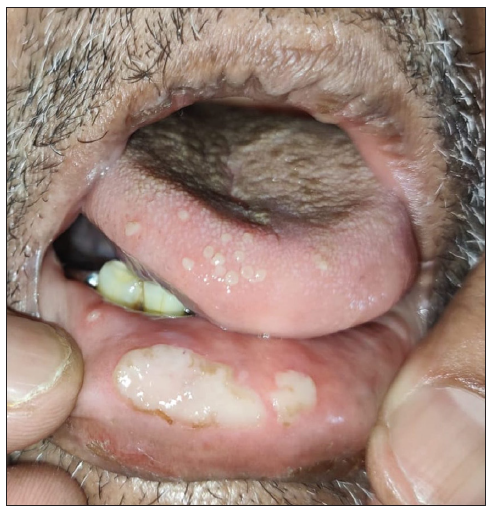
- Polycyclic erosions over lower lip.
- Polycyclic erosions over inner aspect of upper lip.
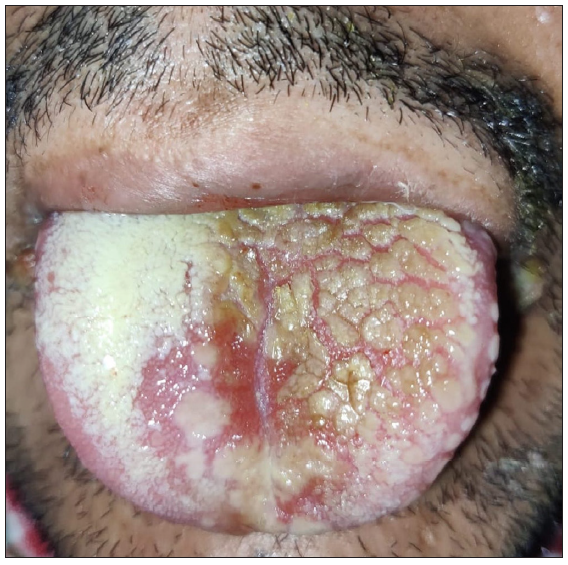
- Yellowish plaque with a cobblestone appearance.
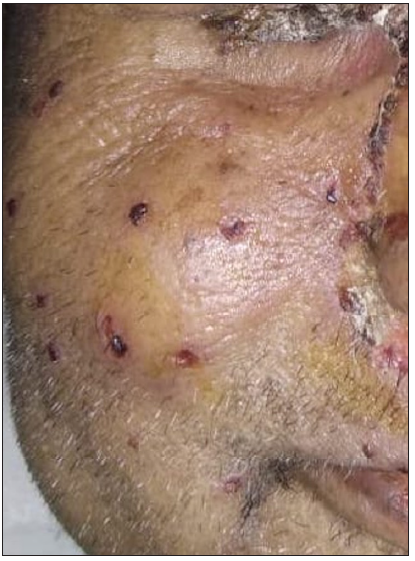
- Monomorphic crusted erosions over cheek.
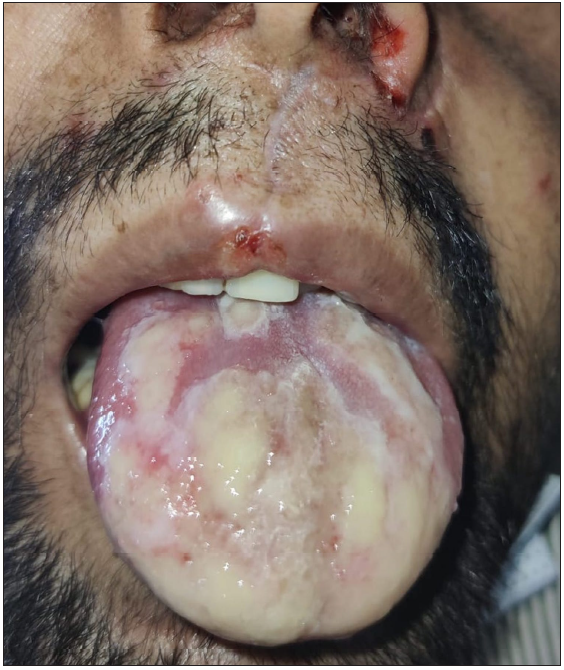
- Large geographic ulcer in oral cavity.
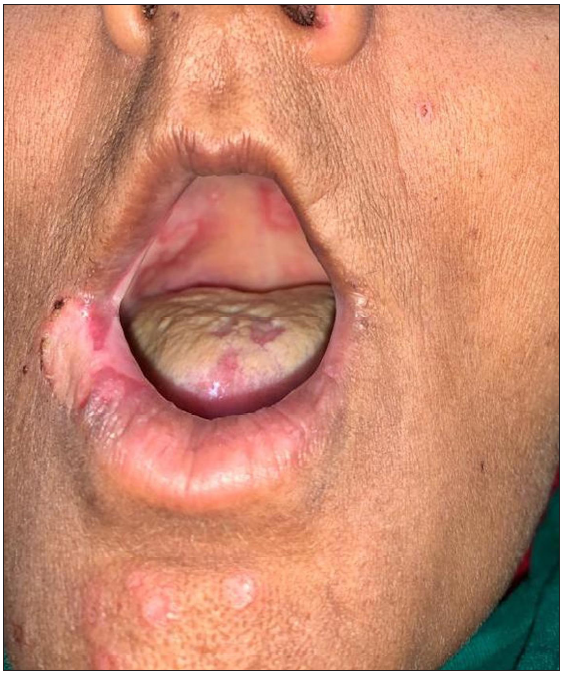
- Polycyclic erosion in the oral cavity with annular erosions on the chin.
In the literature, there are reports of an increased risk of reactivation of the herpes group of infections during and post-COVID-19. Zubchenko et al.2 described the clinical and laboratory features of post-COVID manifestations. Out of 88 patients, 68 were cases and 20 were controls. They found increased reactivation of herpes virus infections in these patients. The detection of viruses was done by PCR on saliva and oral mucosa. Reactivation of Epstein-Barr virus (EBV) was seen in 29 (42.6%), human herpes virus (HHV) - 6 in 17 (25%), and Epstein-Barr virus (EBV) plus HHV- 6 in 22 (32.4%) of these cases. In a study, there was an increased incidence of herpes zoster (HZ) during the COVID-19 pandemic which increased by 35.4% during the pandemic when compared to the same interval in the pre-pandemic period i.e. an average increase of over 10.7 cases per million Brazilian inhabitants during the COVID-19 pandemic.3,4 Similarly, in another questionnaire-based online survey, COVID-19-related herpes simplex virus (HSV) reactivation was more severe in 42.86% (12/28) of patients.5
The proposed mechanisms for HSV reactivation in COVID-19 (12/28) patients were immune dysregulation, COVID-19-related psychological and physical stress, and fever.5 Immune dysregulation caused by COVID-19, mucormycosis, and diabetes mellitus might have been the predisposing factors for herpes simplex reactivation in our patients. Not only the oral mucosa but the face was also involved in four of our patients. The most common presentation was polycyclic erosions in the oral cavity and monomorphic crusted papules on the face. Large geographic ulcers and yellowish plaque with a cobblestone appearance were the atypical presentations. It is important to investigate for herpes in patients with vesicular lesions because there are multiple causes of oral vesicles like autoimmune blistering diseases such as pemphigus vulgaris mucous membrane pemphigoid. Bulla may appear in angina bullosa haemorrhagica and rarely in lichen planus; therefore, differentiating these entities is important for the management of the disease.6 Also, if herpes lesions are more extensive and severe, early antiviral therapy leads to faster healing of lesions and decreases pain. Primary HSV-1 infection may rarely lead to widespread vesicular eruptions, non-healing persistent erosions, or disseminated visceral disease in the immunocompromised patients.7 In our patients, Tzanck smear was positive in 57.1% (4/7) patients. Molecular tests like PCR and viral cultures were not done because of the non-availability of these tests in a resource-poor setting. Another limitation was that the follow-up and prognosis of patients were not assessed.
The present study highlights the atypical clinical presentation of herpetic infection within an epidemic of COVID-19-associated mucormycosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15:102146.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Herpesvirus infections and post-COVID-19 manifestations: A pilot observational study. Rheumatol Int. 2022;42:1523-30.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Increased number of herpes zoster cases in brazil related to the COVID-19 pandemic. Int J Infect Dis. 2021;104:732-3.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Herpes zoster and COVID-19 infection: A coincidence or a causal relationship? Infection. 2022;50:289-93.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- COVID-19 and herpes simplex virus infection: A cross-sectional study. Cureus. 2021;13:e18022.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Oral lesions in autoimmune bullous diseases: An overview of clinical characteristics and diagnostic algorithm. Am J Clin Dermatol. 2019;20:847-61.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Management of oral herpes simplex virus infections: The problem of resistance. A narrative review. Oral Dis 2023 Jun 6
- [CrossRef] [PubMed] [Google Scholar]





