Translate this page into:
Hidradenitis Suppurativa: A Systematic Review and Meta-analysis of Therapeutic Interventions
2 Department of Trauma and Orthopaedic Surgery, Montpellier University Hospital, Montpellier, France
3 Department of Plastic and Reconstructive Surgery, Wound Healing and Burns Units, Lapeyronie University Hospital, Montpellier, France
Correspondence Address:
Huidi Tchero
Unit of Wounds and Healing, Department of Trauma and Orthopaedic Surgery, Centre Hospitalier Louis Constant Fleming Saint Martin, Saint Martin, Guadeloupe
France
| How to cite this article: Tchero H, Herlin C, Bekara F, Fluieraru S, Teot L. Hidradenitis Suppurativa: A Systematic Review and Meta-analysis of Therapeutic Interventions. Indian J Dermatol Venereol Leprol 2019;85:248-257 |
Abstract
Hidradenitis suppurativa is a chronic inflammatory condition that affects skin regions bearing apocrine glands. Although hidradenitis suppurativa is difficult to treat and cure, the currently available treatments are directed toward managing the lesions and associated symptoms. This review presents an evidence-based outline of the available treatment options. We searched four electronic databases and extracted data from retrieved studies for qualitative or quantitative analysis. Meta-analysis was conducted using the comprehensive meta-analysis software to generate pooled standardized mean differences or risk ratios. Numerous medical treatments are available for hidradenitis suppurativa such as antibiotics, retinoids, antiandrogens, immunosuppressive and anti-inflammatory agents and radiotherapy for early lesions. Adalimumab, an anti-tumor necrosis factor antibody, was superior to placebo in reducing Sartorius score (standardized mean difference = −0.32, confidence interval [−0.46, −0.18], P < 0.0001) and pain (risk ratio = 1.42, confidence interval [1.07, 1.9], P = 0.02), when given weekly (not every other week). Combination therapies (such as antibiotics and hyperbaric oxygen therapy) have been tested, which have shown promising results that are yet to be confirmed. Based on the quality of evidence, the most recommended treatments for hidradenitis suppurativa include adalimumab and laser therapy. Surgery (either by simple excision or complete local excision followed by skin graft) is the first choice for intractable disease presenting in the late stages. However, the evidence on most of these treatments is deficient and further randomized trials are needed to establish the most efficient therapies for hidradenitis suppurativa management.
Introduction
Hidradenitis suppurativa, also known as acne inversa or Verneuil's disease, is a chronic inflammatory condition affecting skin regions bearing apocrine glands.[1] The deep-seated, inflamed and painful lesions develop as sinus tracts, nodules or abscesses, most commonly after puberty. The flares that may subside untreated within two weeks occur at varying intervals with painful and suppurative manifestations.[2]
Though it was believed to be a rare disease, the findings of several studies contradicted this view. A survey showed a point prevalence of 1% of the French population.[3] In another study, the prevalence was found to be 4% in young adults undergoing screening for sexually transmitted diseases.[4] This condition shows a significant gender bias with three times more occurrence in women. Although the condition is recorded routinely in postmenopausal women and children, the early 20s is the most common age of affection.[5]
Genetic and hereditary factors play a role in increasing the risk of hidradenitis suppurativa as one-third of the patients report family history.[6] Further, the affected families have been demonstrated to exhibit an autosomal dominant inheritance of the condition. Mutations in γ-secretase complex genes have been linked with a subset of hidradenitis suppurativa, accompanied with a severe form of acne.[7] Obesity is a major risk factor for hidradenitis suppurativa as majority of the patients are overweight.[8] Smoking is also another major risk factor.[9]
Some of the symptoms such as stinging, burning, pain, pruritis, hyperhidrosis and heat are experienced from about 2 days before the appearance of nodules in about half of the patients. A nodule lasts for 1–2 weeks and may remain blind (not burst and subside without intervention or remain silent). But most of the nodules turn into abscesses and drain out. Compared with other chronic dermatological conditions such as psoriasis, the lifestyle of hidradenitis suppurativa patients is heavily affected because of the substantial negative effects of the condition.[10],[11] These patients usually use more sick leaves and score low on the self-reported level of health status scales.[10],[12]
Several other dermatological and nondermatological conditions such as inflammatory bowel disease, sinusitis, acne, palmoplantar pustulosis, hyperosteosis, osteitis—also known as SAPHO syndrome—pyoderma gangrenosum, Adamantiades-Behçet disease, spondyloarthropathy, keratitis-ichthyosis-deafness syndrome, Down's syndrome, squamous cell carcinoma, adenocarcinoma, acne conglobata, severe acne and pilonidal cysts are associated with hidradenitis suppurativa. It is also common that these conditions are misdiagnosed in hidradenitis suppurativa patients.[1]
Clinical evaluation is the most reliable method for diagnosis of hidradenitis suppurativa. Physical examination reveals the typical signs of noninflamed or inflamed nodules; sinuses that may be draining or non-draining; and abscesses in anogenital, inguinal and/or axillary regions.[13] In refractory or atypical cases, bacteriological cultures and biopsies are ordered to guide therapy. Bacteriological examinations show no underlying organisms in almost all cases. However, the presence of a superinfection or various bacteria including Staphylococcus aureus is associated with severity of symptoms.[14],[15] In case of preparing for surgical interventions, ultrasonography is used to delineate the extent of spread of lesions under the skin.[16].
Owing to considerable similarities with other common dermatological conditions such as furunculosis, bacterial folliculitis and inflamed epidermoid cyst, the disease is ?? diagnosed and treated for a longer period of time. Some studies have reported this period to be even up to 12 years.[17] Further, clinicians also tend to treat the condition as common boils with antibiotics or lancing. Antibiotics tend to relieve the inflammation in most cases as the flares are mostly caused by bacterial infection. However, the disease keeps progressing. Although hidradenitis suppurativa is difficult to treat., the tavailable options are directed toward management of the condition and regression of precipitating factors.
The choice of treatment depends on the stage of the condition. As a rule, topical therapy is preferred for stage 1 with necessary systemic treatments prescribed based on the extent of the lesions. The available medical treatments, as assessed in this study, include antibiotics, antiinflammatory, immunosuppressive agents, botulinum toxin, isotretinoin and antiandrogens. Extensive clinical evidence, particularly using randomized controlled design, is scarce on the medical management of hidradenitis suppurativa. Therefore, choices are mostly guided by the clinicians understanding and experience, as well as published case reports and case series.
We performed this systematic review and meta-analysis to investigate the safety and efficacy of available treatment options (medical, radiation and surgical) for hidradenitis suppurativa with published data in the literature.
Methods
Literature search
A systematic literature search according to the PRISMA guidelines was conducted to identify studies on the treatment of patients with hidradenitis suppurativa. A combination of the following terms was used to search PubMed, Scopus, ISI Web of Science and Cochrane CENTRAL: (”acne inversa” OR “hidradenitis suppurativa”) AND (“drug therapy” OR (rifampicin OR moxifloxacin OR metronidazole OR clindamycin OR isotretinoin OR adalimumab OR etanercept OR infliximab OR anakinra) OR (surgery OR laser OR photodynamic therapy). The search results were screened and categorized according to the type of study design such that results from case reports, case series and single-arm retrospective clinical studies were narratively summarized, while data from randomized clinical trials were pooled in a meta-analysis model. The final database search was conducted on January 26, 2018 to include all the suitable studies published in English from inception to search date.
Eligibility criteria
For a study to be meta-analyzed, certain eligibility criteria were applied. The study must be a randomized clinical trial or a comparative clinical trial that compared hidradenitis suppurativa treatments including antibiotics, tumor necrosis factor-α with placebo or another active agent. Two reviewers screened all the retrieved results and in case of discrepancy, the disagreement was solved by discussion with a third reviewer.
Quality assessment
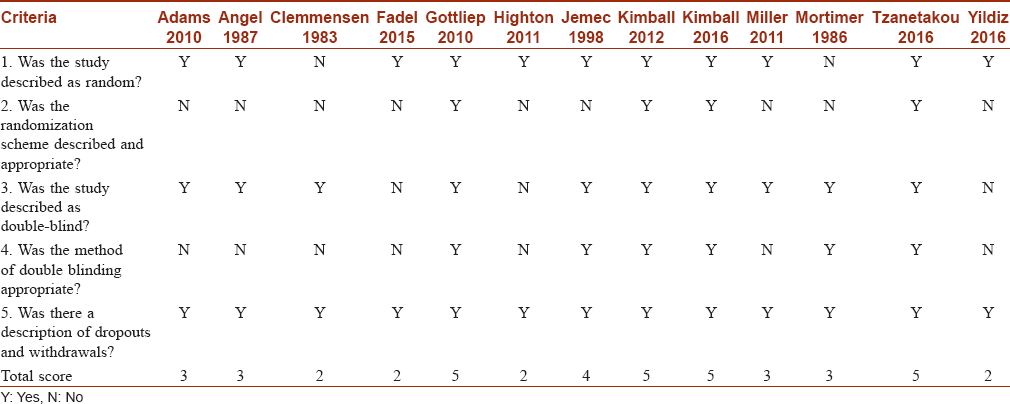
The quality of the included studies was assessed using a 5-point JADAD scale for scoring clinical trials. A positive response for the question was scored 1, whereas a negative response was scored 0. The study was labeled as “low quality” if the total score was ≤2, and as “high quality” if the total score was ≥3.[18]
Data extraction and synthesis
Data were extracted from the studies which met the eligibility criteria and analyzed using comprehensive meta-analysis (version 3). Continuous data were pooled as standardized mean difference and 95% confidence interval, and dichotomous data were pooled as risk ratio and 95% confidence interval. The data extraction was conducted independently by two authors, followed by resolution of discrepancies with mutual discussion. Data that were not suitable for quantitative analysis were analyzed in a qualitative approach.
Results
Literature search results and quality assessment results
Database searching resulted in 544 unique records. After screening these records, 13 randomized trials were found eligible for meta-analysis [Figure - 1].[19],[20],[21],[22],[23],[24],[25],[26],[27],[28],[29],[30],[31] Regarding quality assessment, except for four studies, all the trials were of high quality [Table - 1].
 |
| Figure 1: PRISMA flow diagram of search results and screening |
Outcomes of assessed interventions
Antibiotics
In a small randomized controlled trial, using topical clindamycin was found to diminish the nodules, pustules and abscesses.[21] However, this 3-month study had not assessed the stage of disease and used a 10 mg per milliliter preparation of clindamycin. Oral antibiotics are prescribed as a second-line therapy when desired results are not obtained by topical therapies. Agents with immunomodulatory and antiinflammatory properties are preferred if available.[32] However, a 3-month randomized controlled trial showed no superiority for oral tetracycline compared with topical clindamycin.[24] In a double-blind randomized placebo-controlled trial of patients with stage I or mild stage II hidradenitis suppurativa, a 0.1% clindamycin topical preparation showed favorable effects in terms of patients' assessments, number of abscesses, inflammatory nodules and pustules at each monthly evaluation (P < 0.01).[21]
There is emerging evidence that combination therapies may also be effective. A randomized controlled trial using hyperbaric oxygen therapy as an adjunct to systemic antibiotic therapy (rifampicin and clindamycin) found the combination to be significantly superior to antibiotics alone in improving hidradenitis suppurativa symptoms.[30] The treatment group was administered a hyperbaric oxygen therapy session of 120 min, compression for 20 min, followed by treatment at 2.4 atmospheres absolute for three times (with 5-min air break), each lasting 25 min, followed by 15 min of decompression. A total of 20 sessions were conducted with five sessions every week. The comparison group was given a combination of clindamycin (300 mg orally, BID) and rifampicin (300 mg orally, BID) for 10 weeks. After follow-up for 10 weeks, both Sartorius score and Dermatology Life Quality Index improved in the combination group as compared with antibiotic monotherapy. Nevertheless, further evidence is needed to establish the benefits of adjunct therapies before introducing them in clinical practice.
Antiinflammatory agents
Intralesional injections of glucocorticoids have also shown promising results. Triamcinolone has been found effective at 2–5 mg/ml when used for individual lesions.[1] Recently, a double-blind, randomized trial on the efficacy of anakinra (which inhibits binding of inflammatory pathway mediator interleukin-1 to its receptor) showed that anakinra was effective in reducing the severity of hidradenitis suppurativa.[29] Anakinra was administered subcutaneously every day for 12 weeks at a dose of 100 mg, followed by a 12-week follow-up. Clinical response was achieved in 78% of the subjects (compared with 30% in the placebo group). Further, the disease activity score was significantly lowered by 67% in the anakinra group (compared with 20% in the placebo group).
Antiandrogens
On the basis of anecdotal evidence, clinicians have been using antiandrogens in women with hidradenitis suppurativa.[33] A cross-over double-blind trial in 25 women showed that ethinyl estradiol alone or in combination with cyproterone showed similar benefits in terms of reduction in discharge, lumps, nodules, pain and discomfort. The patients received the drugs as per the reversed sequential regimen of Hammerstein and Cupceancu, wherein a 100 mg of cyproterone acetate per day for 10 days and 50 μg per day of ethinyl estradiol per day for 21 days was administered. After 3–7 months, the ethinyl estradiol dose was reduced to 30 μg per day and the cyproterone dose was reduced to 50 mg per day.
Further, the disease either significantly improved or completely cleared in half of the patients and the number of serious adverse events was significantly lower in the combination group. 28 Antiandrogens such as finasteride have been tested in some small studies. Farrell et al. (two subjects), Joseph et al. (seven subjects) and Domenech et al. (one subject) showed that finasteride was able to achieve a favorable effect, ranging from partial to complete resolution.[34],[35],[36] The dose of finasteride in these studies was 5 mg/day. In the study by Joseph et al., the follow-up periods ranged between 8 and 24 months and patients experienced remissions lasting 8–18 months. The patient in the case report by Domenech et al. experienced complete remission after 1 year of treatment by finasteride. The exact mechanism of action of antiandrogens in achieving resolution of hidradenitis suppurativa is still not clearly understood.
Isotretinoin
Isotretinoin has been found to be less effective. Studies have used a dose of 0.5–1.2 mg/kg daily, administered over 4–12 months. A 4-month intervention with isotretinoin improved lesions in just one-fourth of participants, most of whom were having mild condition. Therefore, isotretinoin is not favored for the treatment of hidradenitis suppurativa.[37]
Immunosuppressive therapy
Rapid benefits have been reported with the systemic immunosuppressive agent cyclosporine.[38],[39] A case of hidradenitis suppurativa, not responding to long-term antibiotics and ultraviolet B therapy responded to 4.5 mg/kg/day dose of cyclosporine. Favorable results appeared at 4 months wherein the hidradenitis suppurativa lesions healed, and discharging sinuses and pain were diminished. The benefits were maintained for 15 months during which cyclosporine was administered along with broad-spectrum antibiotics.[38] However, these are isolated case reports.
The much-preferred agents for autoimmune diseases, the tumor necrosis factor inhibitors, have also given inconsistent results. In a first of its kind, a double-blind trial with 8 weeks of infliximab or placebo with cross-over switching option for placebo group patients to infliximab reported at least 50% improvement in hidradenitis suppurativa severity index score. A total of 33 patients with moderate-to-severe hidradenitis suppurativa were given infliximab at 5 mg/kg intravenously at weeks 0, 2 and 6. The treatment was well-tolerated and provided additional benefits of pain alleviation and reduction in severity. In contrast, another trial showed that etanercept had no benefits compared with placebo as physician and patient global assessment scores were similar between the groups.[19] Using a randomized, double-blind, placebo-controlled trial design, 20 moderate-to-severe hidradenitis suppurativa patients were administered a twice weekly dose of etanercept at 50 mg for 12 weeks, followed by open-label switching to the same dose for 12 more weeks.
The selective phosphodiesterase-4 inhibitor (Apremilast, 30 mg, twice a day) has been found mildly effective in a series of nine hidradenitis suppurativa patients.[40] The duration of treatment with Apremilast ranged from 2 days to 9 months. During treatment, most patients experienced significant reductions in the visual analog scale for pain and Dermatology Life Quality Index. Apremilast, primarily developed for psoriasis, reduces tumor necrosis factor-α production by directly impacting the cyclic AMP formation. It also reduces interleukin-17 and interleukin-23, and increases interleukin-10 that modulates inflammation. Two case reports showed that reducing interleukin-17 levels with the interleukin-17A antibody (Secukinumab) could be effective in severe hidradenitis suppurativa.[41],[42] Other interleukin-17A antibodies such as brodalumab, which showed robust efficacy in interleukin-17-mediated lesions as psoriasis may show significant benefits in hidradenitis suppurativa and should be investigated.[43]
There is accumulating evidence on the safety and efficacy of adalimumab in hidradenitis suppurativa management. More recently, in a phase 2 randomized double-blind trial, adalimumab weekly dosing was found to have significant efficacy in symptomatic management of 154 hidradenitis suppurativa patients, compared with the placebo group.[25] Moreover, in a subpopulation of the same clinical trial, the majority of women with moderate-to-severe hidradenitis suppurativa showed symptomatic reduction of the disease with a 40 mg adalimumab weekly dose when compared with placebo or fortnightly dosing regimen.[31] In recent phase 3 clinical trials, the safety and efficacy of adalimumab was further demonstrated when administered on a weekly basis with dose reduction for 12 weeks.[26] In a post-hoc analysis, adalimumab was found to reduce pain and depressive symptoms associated with hidradenitis suppurativa over a period of 16 weeks.[44]
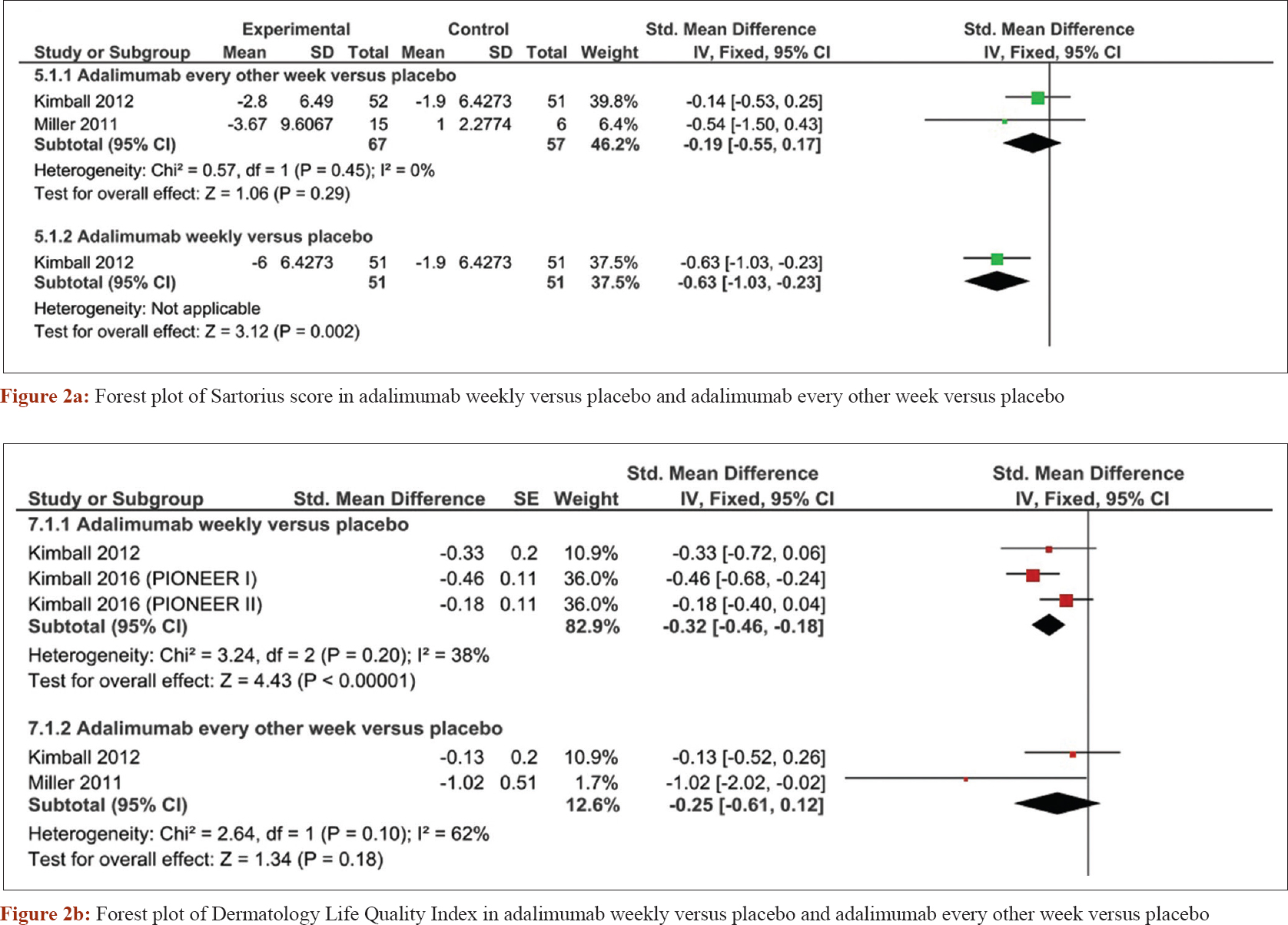
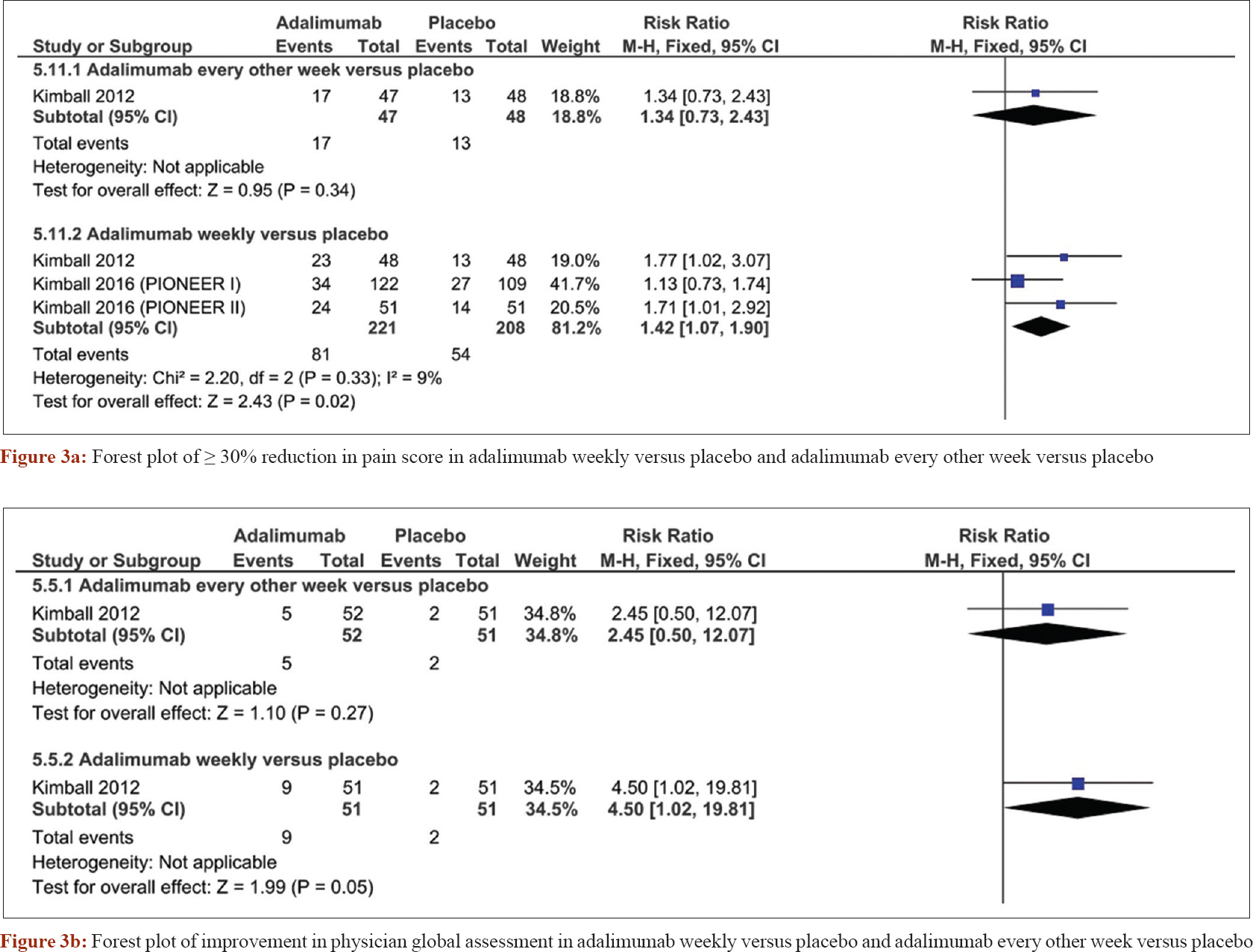
A pooled meta-analysis of four randomized controlled trials[25],[26],[27] was conducted to obtain more precise effect estimates regarding the safety and efficacy of adalimumab. Weekly administration of adalimumab was superior to placebo in terms of decreasing sartorius score (standardized mean difference = −0.32, confidence interval [−0.46, −0.18], P < 0.0001). However, adalimumab administered every other week was not significantly different from placebo (standardized mean difference = −0.25, confidence interval [−0.61, −0.12], P = 0.18) [Figure - 2]a. The Dermatology Life Quality Index analysis showed that adalimumab was superior to placebo when administered weekly (standardized mean difference = −0.63, confidence interval [−1.03, −0.23], P = 0.002), but not when given every other week (standardized mean difference = −0.19, confidence interval [−0.55, 0.17], P = 0.29) [Figure - 2]b. The number of patients with ≥30% reduction in pain score was increased in the adalimumab weekly regimen (risk ratio = 1.42, confidence interval [1.07, 1.9], P = 0.02), but not significantly different with adalimumab given every other week (risk ratio = 1.34, confidence interval [0.73, 2.43], P = 0.34) [Figure - 3]a. Borderline improvement in Physician Global Assessment was observed with the weekly regimen (risk ratio = 4.5, confidence interval [1.02, 19.81], P = 0.05), but not when the drugs was given every other week (risk ratio = 2.45, confidence interval [0.5, 12.07], P = 0.27) [Figure - 3]b.
 |
| Figure 2: |
 |
| Figure 3: |
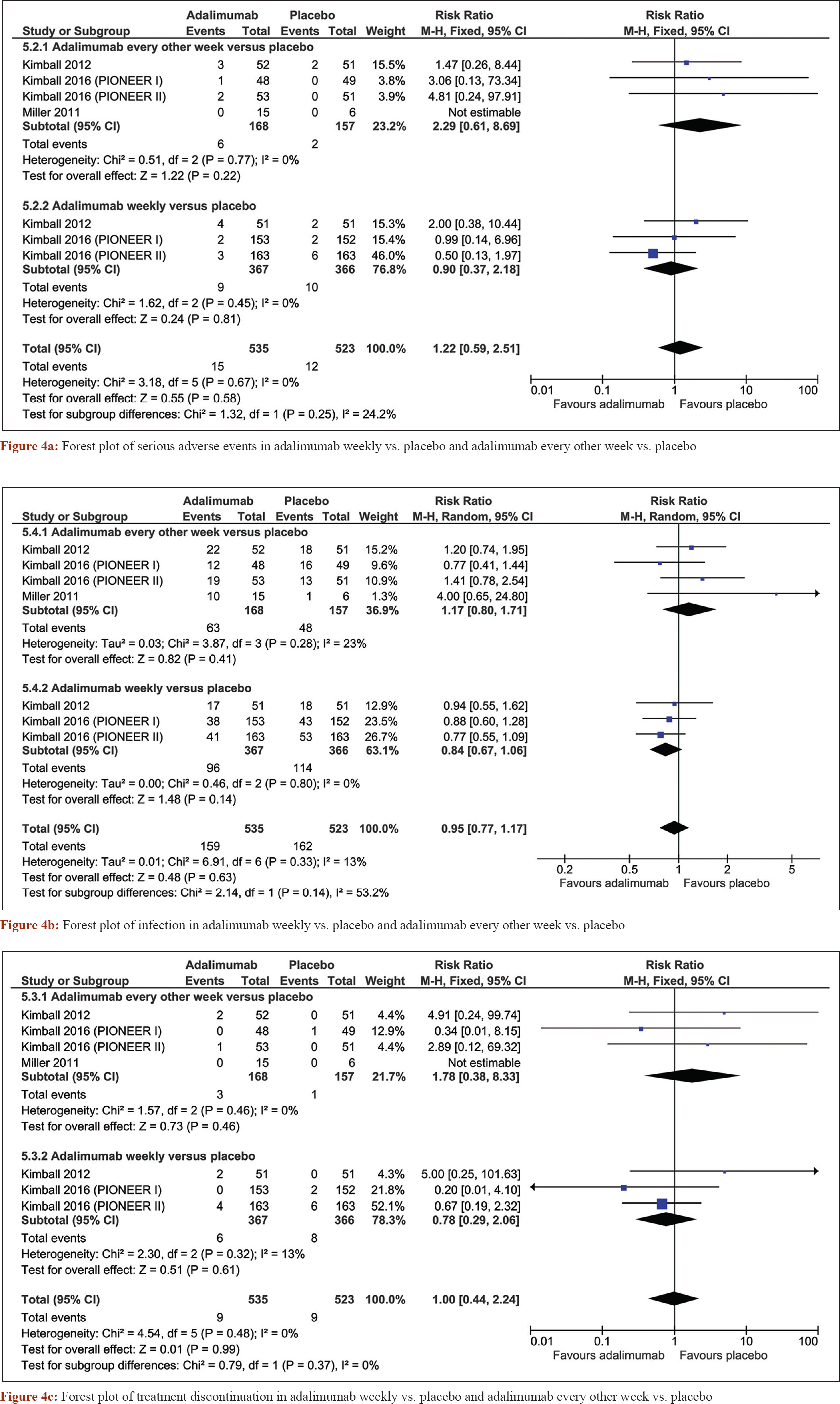
The pooled analysis also showed that adalimumab was highly tolerable. There was no significant difference in the incidence of serious adverse events between adalimumab weekly (risk ratio = 0.9, confidence interval [0.37, 2.18], P = 0.81) and every other week (risk ratio = 2.29, confidence interval [0.61, 8.69], P = 0.22) and placebo [Figure - 4]a. The incidence of infection was similar in both regimens as compared with placebo; weekly (risk ratio = 0.84, confidence interval [0.67, 1.06], P = 0.14) and every other week (risk ratio = 1.17, confidence interval [0.80, 1.71], P = 0.41) [Figure - 4]b. Treatment discontinuation?? was not significantly different between both adalimumab weekly and placebo (risk ratio = 0.78, confidence interval [0.29, 2.06], P = 0.61) nor adalimumab every other week and placebo (risk ratio = 1.78, confidence interval [0.38, 8.33], P = 0.46) [Figure - 5]. Although preliminary, these findings for clinical treatment of hidradenitis suppurativa with adalimumab are promising.
 |
| Figure 4: |
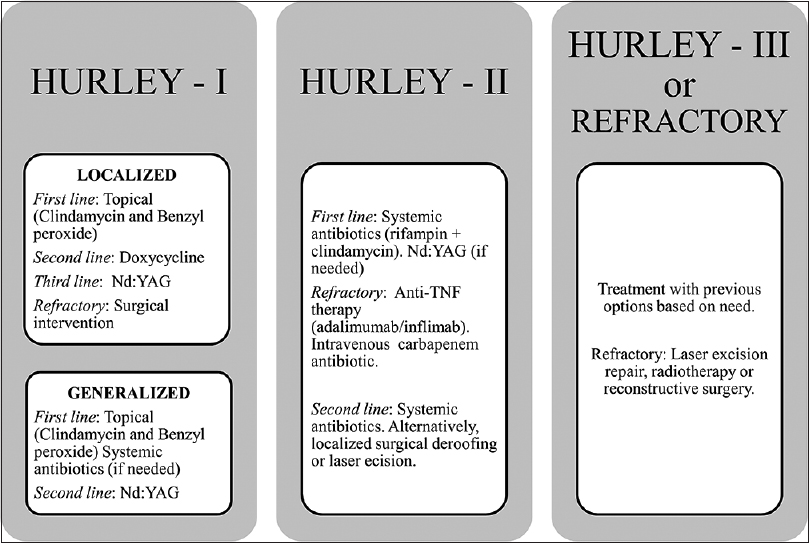 |
| Figure 5: A treatment algorithm based on the evidence from the literature |
Four cases of hidradenitis suppurativa have been treated with botulinum toxin based on the rationale that botulinum toxin reduces sympathetic activation of apocrine glands by lowering the acetylcholine release. Lowered apocrine activity limits inflammation and follicular rupture.[45],[46],[47] These reports used inconsistent regimens (ranging from botulinum toxin administration once to four sessions over 3 years of treatment) with doses ranging between 40 and 50 IU and reported complete remissions after 1–4 doses.
Surgical intervention
Patients with stage 1 and 2 hidradenitis suppurativa do not need surgical interventions. Surgical intervention is preferred as a last option for unresponsive lesions. Extensive scarring invariably needs surgery because it does not respond to medications. Recurrence is very common if the lesions are just drained.[48] And drainage does not work for inflamed and non-fluctuating nodules. In minor surgeries, the roof of the sinus tract is excised, leaving the floor intact to ensure rapid healing.[49] Interestingly, excision of all hair-bearing in the affected region provides much higher benefits compared to excision of just the inflamed lesions.[50] This could be due to the presence of diffuse invisible lesions around the focal lesions.
Laser and radiation therapy
Laser and radiation therapies have been used lately for alleviating hidradenitis suppurativa. A prospective randomized controlled trial on 22 stage 2–3 hidradenitis suppurativa patients were assessed by neodymium-doped yttrium aluminum garnet (Nd:YAG; a hair epilation device) laser. The results showed 65% of all sites, 62% of axillary lesions, 73% of inguinal lesions and 53% of infra-mammary lesions had statistically significant improvement, compared with control patients after 3 months of treatment.[51] In addition to proving the benefits of laser therapy, this study also reinforced the primary follicular pathogenesis of hidradenitis suppurativa. Another study using carbon dioxide laser therapy left the lesions to heal by secondary intention and reported recurrence in just 8% of the lesions after 27 months of follow-up, cementing the long-term benefits of laser intervention.[52]
Highton et al. investigated the efficacy of intense pulsed light versus no treatment for hidradenitis suppurativa.[23] There was no significant difference in terms of the participants global assessment for groin and infra-mammary lesions. However, the treatment was associated with worse assessment for axillary lesions. Another trial by Fadel et al. investigated niosomal methylene blue gel photodynamic therapy versus free methylene blue gel photodynamic therapy for hidradenitis suppurativa.[22] The Hidradenitis Suppurativa Lesion, Area and Severity Index was significantly better in the niosomal methylene blue gel. Staphage lysate was superior to placebo in improving the physician global assessment, as reported by Angel et al.[20]
Discussion
This systematic review discussed the available therapeutic options for hidradenitis suppurativa. We investigated the published literature on several medical, radiation and surgical modalities. Several of these modalities showed good efficacy and tolerability in the included studies. We further performed a meta-analysis of the safety and efficacy of adalimumab in hidradenitis suppurativa patients. The analysis showed that adalimumab is effective when given weekly rather than at every other week. Surgery is reserved for advanced stages, unresponsive cases and extensive scarring.
Compared with general population, hidradenitis suppurativa patients have a 50% higher risk of any type of cancer.[53] These epidemiological studies have also indicated that some cancers such as squamous cell carcinoma, hepatocellular cancer and buccal cancer occur more specifically than others in these patients.[53] However, these findings should be considered with care as smoking was not controlled for in these patients, which is a confounding factor for both the conditions. Finding safe and effective treatments for those patients is therefore necessary.[54]
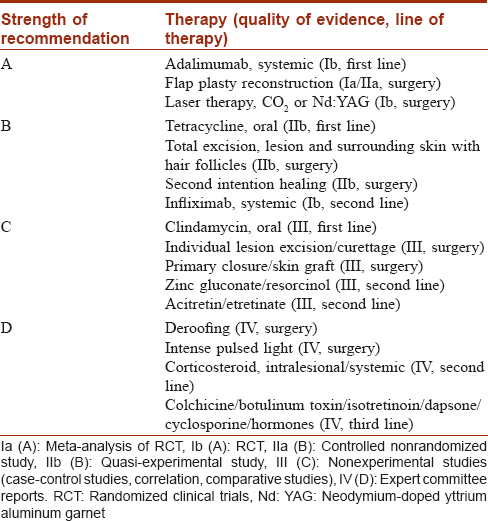
Strength of evidence
The evidence was evaluated as per the GRADE approach for rating the quality of evidence [Table - 2].[55] Further, a treatment algorithm is provided based on our clinical experience and the evidence from this review.
Obscurity and future directions
The limitations of the current study include the low number of available randomized controlled trials in the literature, which prevented meta-analysis for most assessed regimens. Moreover, adequate follow-up information were not reported for several regimens. Currently, there are no set guidelines available for the management of hidradenitis suppurativa. A lot remains to be learnt about hidradenitis suppurativa as randomized controlled trials are to be conducted on various aspects. For instance, the different therapies need to be compared for efficacy, duration of treatment necessary and achieving permanent remission. Similarly, combination therapy needs to be established as a second line of treatment if monotherapy fails. There is also need for undertaking trials on immunosuppressive agents as single treatments as well as in combination with antibiotics. Finally, the effectiveness of surgical techniques and postsurgical management practices remains to be studied well.
Conclusion
In this review, we presented an evidence-based evaluation of hidradenitis suppurativa management modalities. Further, we prepared a treatment algorithm based on the evidence and our own experience at our clinic. Owing to the complex nature of hidradenitis suppurativa, the patient will have a better chance of recovery if diagnosed at early stage, followed by proper treatment, preferably based on staging followed by adherence to evidence-based algorithm. All patients may need one or more of adjuvant therapies to manage associated pain, depression, weight loss and infections.
Numerous medical treatments are available for hidradenitis suppurativa such as antibiotics, retinoids, antiandrogens, immunosuppressive and anti-inflammatory agents and radiotherapy for early lesions. Adalimumab, an anti-tumor necrosis factor antibody, was superior to placebo in reducing Sartorius score and pain, when given weekly (not every other week). Combination therapies (such as antibiotics and hyperbaric oxygen therapy) have been tested and showed promising results that are yet to be confirmed. On the basis of quality of evidence, the most recommended treatments for hidradenitis suppurativa include adalimumab and laser therapy. Surgery (either by simple excision or complete local excision followed by skin graft) is the first choice for intractable disease presenting at late stages. The evidence on most of these treatments is deficient and further randomized controlled trials are needed to establish the most efficient therapies for hidradenitis suppurativa management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Jemec G, Revuz J, Leyden JJ. Hidradenitis Suppurativa. New York; Springer Science and Business Media; 2006.
[Google Scholar]
|
| 2. |
von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2000;14:389-92.
[Google Scholar]
|
| 3. |
Revuz JE, Canoui-Poitrine F, Wolkenstein P, Viallette C, Gabison G, Pouget F, et al. Prevalence and factors associated with hidradenitis suppurativa: Results from two case-control studies. J Am Acad Dermatol 2008;59:596-601.
[Google Scholar]
|
| 4. |
Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol 1996;35:191-4.
[Google Scholar]
|
| 5. |
Dhaou BB, Boussema F, Aydi Z, Baili L, Rokbani L. Hidradenitis suppurativa (Verneuil's disease). J Saudi Soc Dermatol Dermatol Surg 2013;17:1-5.
[Google Scholar]
|
| 6. |
Fitzsimmons JS, Guilbert PR. A family study of hidradenitis suppurativa. J Med Genet 1985;22:367-73.
[Google Scholar]
|
| 7. |
Wang B, Yang W, Wen W, Sun J, Su B, Liu B, et al. Gamma-secretase gene mutations in familial acne inversa. Science 2010;330:1065.
[Google Scholar]
|
| 8. |
Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: A comprehensive review. J Am Acad Dermatol 2009;60:539-61.
[Google Scholar]
|
| 9. |
König A, Lehmann C, Rompel R, Happle R. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology 1999;198:261-4.
[Google Scholar]
|
| 10. |
Esmann S, Jemec GB. Psychosocial impact of hidradenitis suppurativa: A qualitative study. Acta Derm Venereol 2011;91:328-32.
[Google Scholar]
|
| 11. |
Wolkenstein P, Loundou A, Barrau K, Auquier P, Revuz J; Quality of Life Group of the French Society of Dermatology. Quality of life impairment in hidradenitis suppurativa: A study of 61 cases. J Am Acad Dermatol 2007;56:621-3.
[Google Scholar]
|
| 12. |
Jemec GB, Heidenheim M, Nielsen NH. A case-control study of hidradenitis suppurativa in an STD population. Acta Derm Venereol 1996;76:482-3.
[Google Scholar]
|
| 13. |
Parks RW, Parks TG. Pathogenesis, clinical features and management of hidradenitis suppurativa. Ann R Coll Surg Engl 1997;79:83-9.
[Google Scholar]
|
| 14. |
Lapins J, Jarstrand C, Emtestam L. Coagulase-negative staphylococci are the most common bacteria found in cultures from the deep portions of hidradenitis suppurativa lesions, as obtained by carbon dioxide laser surgery. Br J Dermatol 1999;140:90-5.
[Google Scholar]
|
| 15. |
Jemec GB, Faber M, Gutschik E, Wendelboe P. The bacteriology of hidradenitis suppurativa. Dermatology 1996;193:203-6.
[Google Scholar]
|
| 16. |
Wortsman X, Jemec GB. Real-time compound imaging ultrasound of hidradenitis suppurativa. Dermatol Surg 2007;33:1340-2.
[Google Scholar]
|
| 17. |
Mebazaa A, Ben Hadid R, Cheikh Rouhou R, Trojjet S, El Euch D, Mokni M, et al. Hidradenitis suppurativa: A disease with male predominance in Tunisia. Acta Dermatovenerol Alp Pannonica Adriat 2009;18:165-72.
[Google Scholar]
|
| 18. |
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996;17:1-2.
[Google Scholar]
|
| 19. |
Adams DR, Yankura JA, Fogelberg AC, Anderson BE. Treatment of hidradenitis suppurativa with etanercept injection. Arch Dermatol 2010;146:501-4.
[Google Scholar]
|
| 20. |
Angel M, Ramasastry S, Manders E, Ganfield D, Futrell J. Beneficial effects of staphage lysate in the treatment of chronic recurrent hidradenitis suppurativa. Surg Forum 1987;1987:111-2.
[Google Scholar]
|
| 21. |
Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol 1983;22:325-8.
[Google Scholar]
|
| 22. |
Fadel MA, Tawfik AA. New topical photodynamic therapy for treatment of hidradenitis suppurativa using methylene blue niosomal gel: A single-blind, randomized, comparative study. Clin Exp Dermatol 2015;40:116-22.
[Google Scholar]
|
| 23. |
Highton L, Chan WY, Khwaja N, Laitung JK. Treatment of hidradenitis suppurativa with intense pulsed light: A prospective study. Plast Reconstr Surg 2011;128:459-65.
[Google Scholar]
|
| 24. |
Jemec GB, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol 1998;39:971-4.
[Google Scholar]
|
| 25. |
Kimball AB, Kerdel F, Adams D, Mrowietz U, Gelfand JM, Gniadecki R, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: A parallel randomized trial. Ann Intern Med 2012;157:846-55.
[Google Scholar]
|
| 26. |
Kimball AB, Okun MM, Williams DA, Gottlieb AB, Papp KA, Zouboulis CC, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med 2016;375:422-34.
[Google Scholar]
|
| 27. |
Miller I, Lynggaard CD, Lophaven S, Zachariae C, Dufour DN, Jemec GB. Adouble-blind placebo-controlled randomized trial of adalimumab in the treatment of hidradenitis suppurativa. Br J Dermatol 2011;165:391-8.
[Google Scholar]
|
| 28. |
Mortimer PS, Dawber RP, Gales MA, Moore RA. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol 1986;115:263-8.
[Google Scholar]
|
| 29. |
Tzanetakou V, Kanni T, Giatrakou S, Katoulis A, Papadavid E, Netea MG, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: A randomized clinical trial. JAMA Dermatol 2016;152:52-9.
[Google Scholar]
|
| 30. |
Yildiz H, Senol L, Ercan E, Bilgili ME, Karabudak Abuaf O. A prospective randomized controlled trial assessing the efficacy of adjunctive hyperbaric oxygen therapy in the treatment of hidradenitis suppurativa. Int J Dermatol 2016;55:232-7.
[Google Scholar]
|
| 31. |
Gottlieb A, Menter A, Armstrong A, Ocampo C, Gu Y, Teixeira HD. Adalimumab treatment in women with moderate-to-severe hidradenitis suppurativa from the placebo-controlled portion of a phase 2, randomized, double-blind study. J Drugs Dermatol 2016;15:1192-6.
[Google Scholar]
|
| 32. |
Altenburg J, de Graaff CS, van der Werf TS, Boersma WG. Immunomodulatory effects of macrolide antibiotics – Part 1: Biological mechanisms. Respiration 2011;81:67-74.
[Google Scholar]
|
| 33. |
Sawers RS, Randall VA, Ebling FJ. Control of hidradenitis suppurativa in women using combined antiandrogen (cyproterone acetate) and oestrogen therapy. Br J Dermatol 1986;115:269-74.
[Google Scholar]
|
| 34. |
Doménech C, Matarredona J, Escribano-Stablé JC, Devesa JP, Vicente J, Jaén A, et al. Facial hidradenitis suppurativa in a 28-year-old male responding to finasteride. Dermatology 2012;224:307-8.
[Google Scholar]
|
| 35. |
Farrell AM, Randall VA, Vafaee T, Dawber RP. Finasteride as a therapy for hidradenitis suppurativa. Br J Dermatol 1999;141:1138-9.
[Google Scholar]
|
| 36. |
Joseph MA, Jayaseelan E, Ganapathi B, Stephen J. Hidradenitis suppurativa treated with finasteride. J Dermatolog Treat 2005;16:75-8.
[Google Scholar]
|
| 37. |
Boer J, van Gemert MJ. Long-term results of isotretinoin in the treatment of 68 patients with hidradenitis suppurativa. J Am Acad Dermatol 1999;40:73-6.
[Google Scholar]
|
| 38. |
Buckley DA, Rogers S. Cyclosporin-responsive hidradenitis suppurativa. J R Soc Med 1995;88:289P-90P.
[Google Scholar]
|
| 39. |
Rose RF, Goodfield MJ, Clark SM. Treatment of recalcitrant hidradenitis suppurativa with oral ciclosporin. Clin Exp Dermatol 2006;31:154-5.
[Google Scholar]
|
| 40. |
Weber P, Seyed Jafari SM, Yawalkar N, Hunger RE. Apremilast in the treatment of moderate to severe hidradenitis suppurativa: A case series of 9 patients. J Am Acad Dermatol 2017;76:1189-91.
[Google Scholar]
|
| 41. |
Giuseppe P, Nicola P, Valentina C, Elena C, Salvatrice C, Rosario G, et al. Acase of moderate hidradenitis suppurativa and psoriasis treated with secukinumab. Ann Dermatol 2018;30:462-4.
[Google Scholar]
|
| 42. |
Jørgensen AR, Yao Y, Thomsen SF. Therapeutic response to secukinumab in a 36-year-old woman with hidradenitis suppurativa. Case Rep Dermatol Med 2018;2018:8685136.
[Google Scholar]
|
| 43. |
Attia A, Abushouk AI, Ahmed H, Gadelkarim M, Elgebaly A, Hassan Z, et al. Safety and efficacy of brodalumab for moderate-to-severe plaque psoriasis: A systematic review and meta-analysis. Clin Drug Investig 2017;37:439-51.
[Google Scholar]
|
| 44. |
Scheinfeld N, Sundaram M, Teixeira H, Gu Y, Okun M. Reduction in pain scores and improvement in depressive symptoms in patients with hidradenitis suppurativa treated with adalimumab in a phase 2, randomized, placebo-controlled trial. Dermatol Online J 2016;22. pii: 13030/qt38x5922j.
[Google Scholar]
|
| 45. |
Feito-Rodríguez M, Sendagorta-Cudós E, Herranz-Pinto P, de Lucas-Laguna R. Prepubertal hidradenitis suppurativa successfully treated with botulinum toxin A. Dermatol Surg 2009;35:1300-2.
[Google Scholar]
|
| 46. |
Khoo AB, Burova EP. Hidradenitis suppurativa treated with clostridium botulinum toxin A. Clin Exp Dermatol 2014;39:749-50.
[Google Scholar]
|
| 47. |
Martina E, Offidani A. Hidradenitis suppurativa: How to treat with BoNT-A. In: Campanati A, Offidani A, editors. Botulinum Toxin in Dermatology. New York; Nova Science Publishers; 2015. p. 61-75.
[Google Scholar]
|
| 48. |
Ritz JP, Runkel N, Haier J, Buhr HJ. Extent of surgery and recurrence rate of hidradenitis suppurativa. Int J Colorectal Dis 1998;13:164-8.
[Google Scholar]
|
| 49. |
van der Zee HH, Prens EP, Boer J. Deroofing: A tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol 2010;63:475-80.
[Google Scholar]
|
| 50. |
Mandal A, Watson J. Experience with different treatment modules in hidradenitis suppurativa: A study of 106 cases. Surgeon 2005;3:23-6.
[Google Scholar]
|
| 51. |
Tierney E, Mahmoud BH, Hexsel C, Ozog D, Hamzavi I. Randomized control trial for the treatment of hidradenitis suppurativa with a neodymium-doped yttrium aluminium garnet laser. Dermatol Surg 2009;35:1188-98.
[Google Scholar]
|
| 52. |
Finley EM, Ratz JL. Treatment of hidradenitis suppurativa with carbon dioxide laser excision and second-intention healing. J Am Acad Dermatol 1996;34:465-9.
[Google Scholar]
|
| 53. |
Lapins J, Ye W, Nyrén O, Emtestam L. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol 2001;137:730-4.
[Google Scholar]
|
| 54. |
Slade DE, Powell BW, Mortimer PS. Hidradenitis suppurativa: Pathogenesis and management. Br J Plast Surg 2003;56:451-61.
[Google Scholar]
|
| 55. |
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6.
[Google Scholar]
|
Fulltext Views
17,446
PDF downloads
4,126





