Translate this page into:
High prevalence of Mycoplasma genitalium in men who have sex with men: A cross-sectional study
2 Department of Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
3 Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, India
Correspondence Address:
Neena Khanna
Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi
India
| How to cite this article: Dhawan B, Rawre J, Dhawan N, Bhatia R, Gupta V, Khanna N. High prevalence of Mycoplasma genitalium in men who have sex with men: A cross-sectional study. Indian J Dermatol Venereol Leprol 2020;86:195-196 |
Sir,
Mycoplasma genitalium (M. genitalium) and Chlamydia trachomatis (C. trachomatis) are common causes of nongonococcal urethritis (NGU) mainly in men and an increasingly recognized cause of cervicitis and pelvic inflammatory disease (PID) in women. They are also important cofactors for HIV transmission.[1]
Men who have sex with men (MSM) are considered at high risk of sexually transmitted infections (STIs), MSMs are not routinely tested for M. genitalium in India, and therefore there is paucity of data on its prevalence and role in urogenital symptoms in this population.
Syndromic management of urethritis is aimed at treating infections with C. trachomatis and Neisseria gonorrhoeae and consists of therapy with a single oral dose of 1 g of azithromycin and 400 mg of cefexime. Single-dose azithromycin though effective against C. trachomatis is often suboptimal treatment for M. genitalium andtreatment failure is increasingly being reported.
We estimated the prevalence of M. genitalium and its co-occurrence with C. trachomatis, in MSMs attending the STI Clinic of Department of Dermatology and Venereology at All India Institute of Medical Sciences, New Delhi.
All consecutive MSMs presenting with symptoms of urethritis to the STI Clinic of our hospital between January 2017 and April 2018 were enrolled for the study. “Urethritis” was defined as patients having a recent history of dysuria, urethral discomfort, or urethral discharge on examination; ≥neutrophils per high-power field on urethral gram stain.[1]
First-void urine and rectal samples were collected routinely for chlamydia polymerase chain reaction (PCR) targeting cryptic plasmid.[2] In addition, oropharyngeal swabs were also collected if the patient gave history of orogenital sex. The residual DNA of these samples was retrieved and used for detection of M. genitalium using an “in-house” PCR targeting the MgPa gene [Figure - 1].[3] This study was conducted with the approval of the institute research ethics committee.
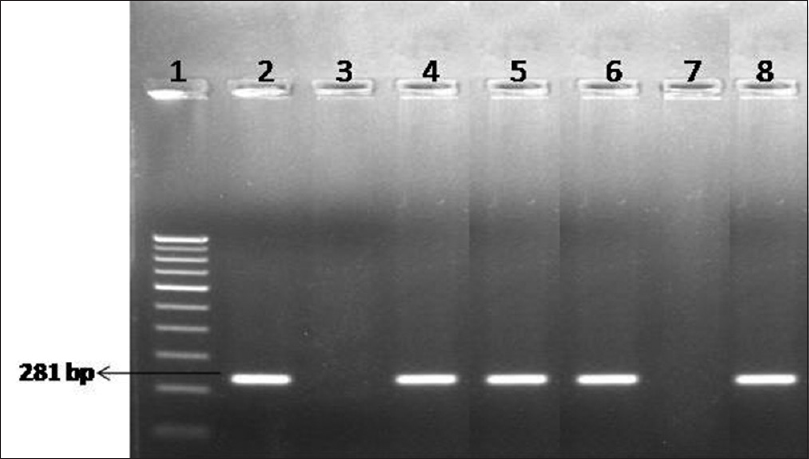 |
| Figure 1: Agarose gel electrophoresis for detection of Mycoplasma genitalium by polymerase chain reaction. Lane 1: 100-bp DNA ladder; Lane 2: positive control; Lane 3: negative control; Lane 4–6: clinical samples – positive; Lane 7: clinical sample – negative; Lane 8: clinical sample – positive |
A total of 99 samples collected from 46 MSMs during the study period were included in the analysis. Specimens included urine (n = 46), rectal swabs (n = 46), and pharyngeal swabs (n = 7). Of the 46 patients tested, 21 (45.6%) were seropositive for human immunodeficiency virus-1 (HIV-1).
A total of 19 (41.3%) patients were positive for M. genitalium infection. M. genitalium positivity rate varied according to anatomical site. The anorectum was the most commonly infected site (13/19; 68.4%) followed by the urethra (9/19; 47.4%). Three of these patients had infections of both the areas. No oropharyngeal M. genitalium infections were detected. Half of the anorectal infections were asymptomatic.
A total of 15 (32.6%) patients were positive for C. trachomatis infection. C. trachomatis positivity rate varied according to anatomical site with the detection of C. trachomatis in 8 of 15 (53.3%) rectal, 10 of 15 (66.7%) urethral, and 1 of 7 (14.2%) at oropharyngeal sites. Of the 8 patients who tested positive for C. trachomatis at the anorectal site, 5 (62.5%) did not have concomitant urethral infection.
Of the 46 MSMs diagnosed with urethritis, 14 (30.4%) were infected with M. genitalium, 10 (21.7%) with C. trachomatis, and 5 (10.8%) were coinfected with both M. genitalium and C. trachomatis. M. genitalium was more prevalent than C. trachomatis (41.3% vs 32.6%; P = 0.03) and was significantly associated with HIV positivity (10/19; 52.6%) in contrast to C. trachomatis (3/15; 20.0%) (P = 0.03).
M. genitalium was first isolated in the early 1980s in men with NGU. The infective profile of M. genitalium has not been thoroughly highlighted because of the difficulties in detecting the microorganism by culture. Molecular techniques have revolutionized sexually transmitted infection testing. The diagnosis of M. genitalium is exclusively based on PCR technology.
We were unable to find any previous reports from India assessing the prevalence of M. genitalium in MSMs. When compared with studies from other countries, our rate of M. genitalium infection was higher. In clinic-based surveys in MSMs, prevalence estimates varied widely from 0.6% to 12.6%. M. genitalium was detected in 21.0% of MSMs with complaints of urethritis indicating that amplification assay detecting M. genitalium should be considered in the sexually transmitted infection management protocols as an important pathogen, particularly in MSMs.[4] Previous studies have shown a strong association between M. genitalium and HIV infection, therefore screening and treatment for M. genitalium has been suggested as part of HIV prevention strategies.[5] We also observed much higher rates of M. genitalium infection in HIV-positive MSMs when compared with HIV-negative MSMs (47.6% vs 36.0%; P = 0.04).
The prevalence of C. trachomatis in MSMs in our study was 15%. Studies in MSMs have estimated prevalence of C. trachomatis ranging from 3.0% to 13.0%.[6],[7] Within studies that tested for both pathogens, prevalence estimates for M. genitalium and C. trachomatis weresimilar in Great Britain, but higher for C. trachomatis than M. genitalium in Denmark and the United States.[4]
We did not perform macrolide resistance testing of M. genitalium which may have added further insights on the utility of prescribing macrolides, one of the recommended first-line options for NGU. Future work should assess the need for appropriate screening and treatment of M. genitalium infection in MSM, particularly those with HIV infection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Pond MJ, Nori AV, Witney AA, Lopeman RC, Butcher PD, Sadiq ST, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis 2014;58:631-7.
[Google Scholar]
|
| 2. |
Mahony J, Chong S, Jang D, Luinstra K, Faught M, Dalby D, et al. Urine specimens from pregnant and nonpregnant women inhibitory to amplification of chlamydia trachomatis nucleic acid by PCR, ligase chain reaction, and transcription-mediated amplification: Identification of urinary substances associated with inhibition and removal of inhibitory activity. J Clin Microbiol 1998;36:3122-6.
[Google Scholar]
|
| 3. |
Jensen JS, Uldum SA, Søndergård-Andersen J, Vuust J, Lind K. Polymerase chain reaction for detection of Mycoplasma genitalium in clinical samples. J Clin Microbiol 1991;29:46-50.
[Google Scholar]
|
| 4. |
Baumann L, Cina M, Egli-Gany D, Goutaki M, Halbeisen FS, Lohrer GR, et al. Prevalence of Mycoplasma genitalium in different population groups: Systematic review and meta-analysis. Sex Transm Infect 2018;94:255-62.
[Google Scholar]
|
| 5. |
Bissessor M, Tabrizi SN, Bradshaw CS, Fairley CK, Hocking JS, Garland SM, et al. The contribution of Mycoplasma genitalium to the aetiology of sexually acquired infectious proctitis in men who have sex with men. Clin Microbiol Infect 2016;22:260-5.
[Google Scholar]
|
| 6. |
Family Health International. The Asian Epidemic Model: Projections of HIV/AIDS in Thailand 2005-2025. Bangkok: Family Health International; 2008.
[Google Scholar]
|
| 7. |
Hernandez AL, Lindan CP, Mathur M, Ekstrand M, Madhivanan P, Stein ES, et al. Sexual behavior among men who have sex with women, men, and Hijras in Mumbai, India – Multiple sexual risks. AIDS Behav 2006;10:S5-16.
[Google Scholar]
|
Fulltext Views
3,604
PDF downloads
1,916





