Translate this page into:
High-risk human papillomavirus in ruxolitinib-associated sebaceous neoplasms
Corresponding author: Dr. Ibrahim Khalifeh, Department of Pathology, American University of Beirut Medical Center, P.O. Box 11-0236, Riad El Solh 1107 2020, Beirut, Lebanon. ik08@aub.edu.lb
-
Received: ,
Accepted: ,
How to cite this article: Hamie L, Bardawil T, Khalifeh I. High-risk human papillomavirus in ruxolitinib-associated sebaceous neoplasms. Indian J Dermatol Venereol Leprol 2021;87:404-8.
Sir,
Ruxolitinib is a JAK1/2 inhibitor approved for polycythemia vera and myelofibrosis in adults. Cutaneous malignancies, namely, nonmelanoma skin cancers, such as squamous cell carcinomas, basal cell carcinomas and Merkel cell carcinoma, have been reported with its use.1,2 In fact, the 5-year efficacy data on ruxolitinib has revealed a significantly higher rate of new-onset basal or squamous cell carcinomas compared to patients on other therapies for myelofibrosis such as hydroxyurea. In addition, these tumors tend to display more aggressive biological behavior and metastatic potential.1,3
The clinical efficacy of ruxolitinib is measured through the elimination of neoplastic cells with the JAK2 mutation.2JAK1/2 inhibitors work by inhibiting proinflammatory cytokines, chemokines and adhesion molecules which can lead to the interruption of certain immune/inflammatory cascades. Despite its obvious benefits, ruxolitinib might push patients towards a relatively immune-suppressed state.2 On extensive review of the English literature, we were unable to find cases of ruxolitinib-induced benign sebaceous tumors with a possible relationship to a high-risk human papillomavirus strain.
A 57-year-old male with myelofibrosis, maintained on ruxolitinib over the past year presented to the dermatology clinic at the American University of Beirut Medical Center, complaining of two growing nodules on the scalp. The bigger lesion was of 5-month duration and the smaller one of 2-month duration. The lesions were asymptomatic; hence, no treatment was sought. On the frontal scalp, there were a 3 × 2 cm and a 1 × 1 cm, skin-colored, firm, oval plaques with areas of hyperkeratosis [Figures 1a and 1b]. Our differential diagnoses included squamous cell carcinoma, keratoacanthoma and adnexal tumors.
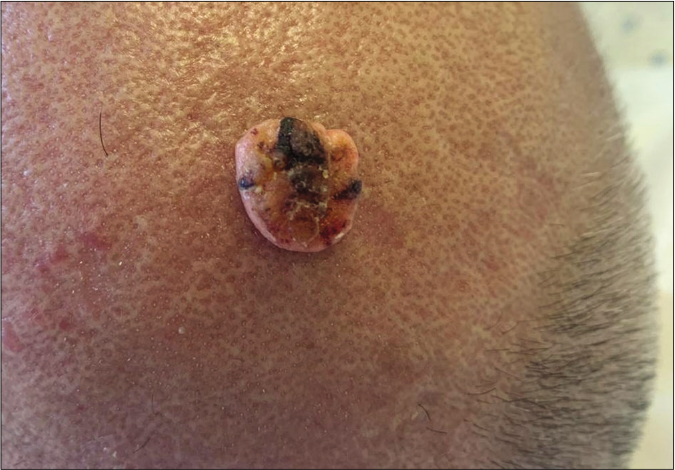
- A skin-colored to yellowish firm nodule with central hyperkeratosis located on the anterior scalp
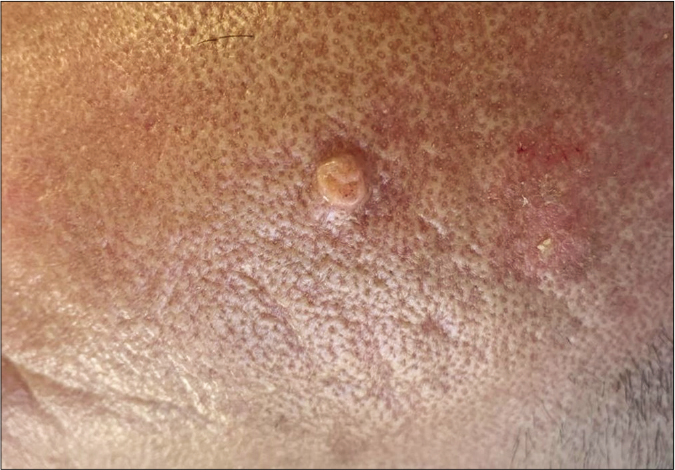
- A skin-colored papule with an elevated border and central depression
On microscopy, both lesions were well-circumscribed with different proportions of sebocytes and germ cells. Both lesions had papillomatous epidermal proliferations with koilocytic changes and mounds of parakeratosis admixed with hemorrhage at the top of the papillae, simulating the diagnosis of verruca vulgaris [Figures 2a - 2d]. The lesions were diffusely immunoreactive to the androgen receptor (nuclear) and epithelial membrane antigen (cytoplasmic/membranous) confirming their sebaceous nature [Figures 2e - 2h]. Consequently, the bigger lesion had more than 50% germ cells and the smaller one had predominantly mature sebocytes; hence, the tumors were diagnosed as a sebaceoma and a sebaceous adenoma, respectively. The patient underwent surgical excision of the lesions and therapy with ruxolitinib was continued.
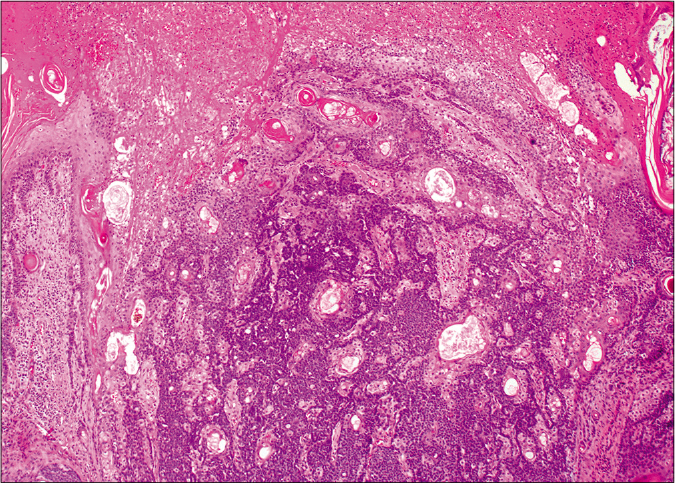
- Basaloid germ cells (80%) admixed with mature sebocytes (20%). The top of the lesion shows mounds of parakeratosis and hemorrhage (H and E,×20)
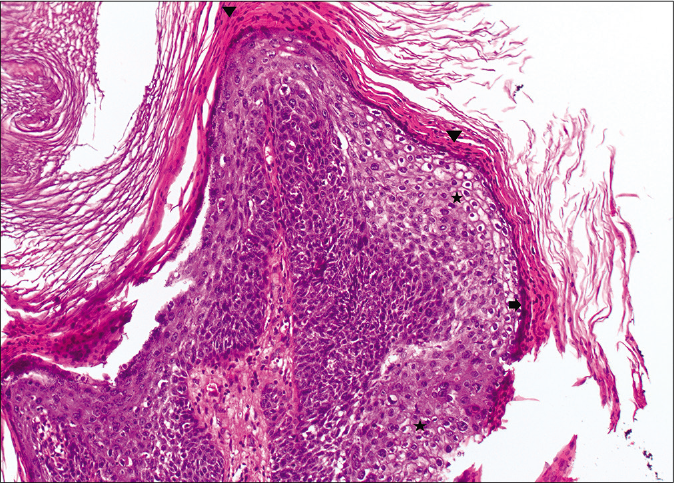
- Papillary configurations lined by squamous cells with koilocytic changes (star) and hypergranulosis (arrow) with overlying parakeratosis (arrowhead) (H and E,×10)
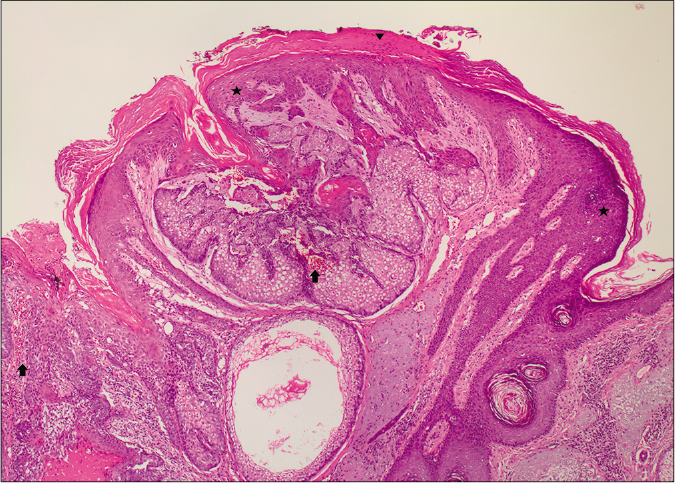
- A well-circumscribed lesioncomposed of mature sebocytesand a few germ cells.The epidermis shows mounds of parakeratosis (arrowhead), hemorrhage (arrow) and koilocytes (star) (H and E,×20)
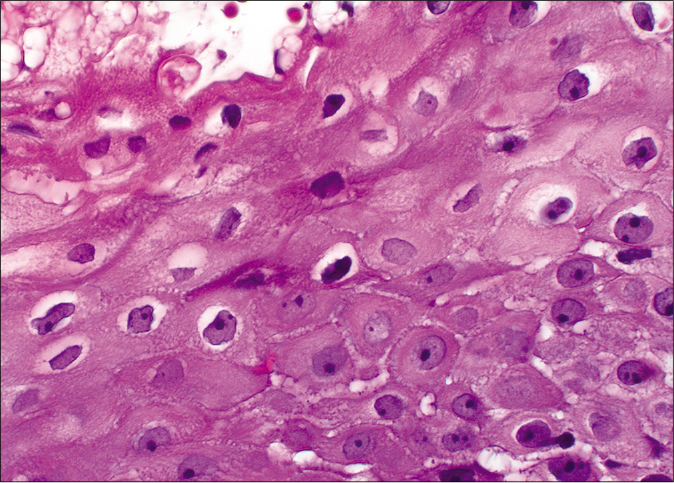
- Koilocytic cells with enlarged pyknotic, wrinkled nuclei with a surrounding halo (H and E,×40)
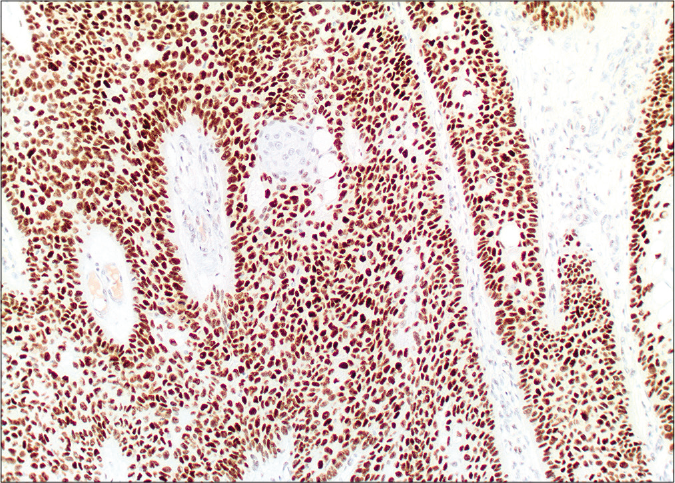
- Diffuse nuclear labelling by androgen receptor antibody (×10)
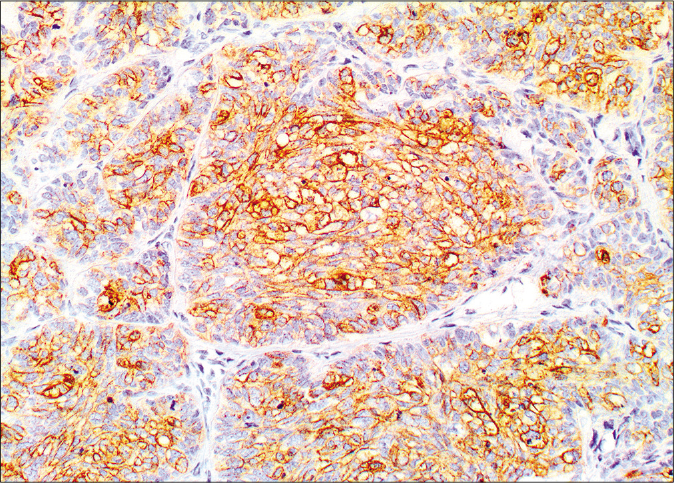
- Membranous and cytoplasmic staining by epithelial membrane antigen (×20)
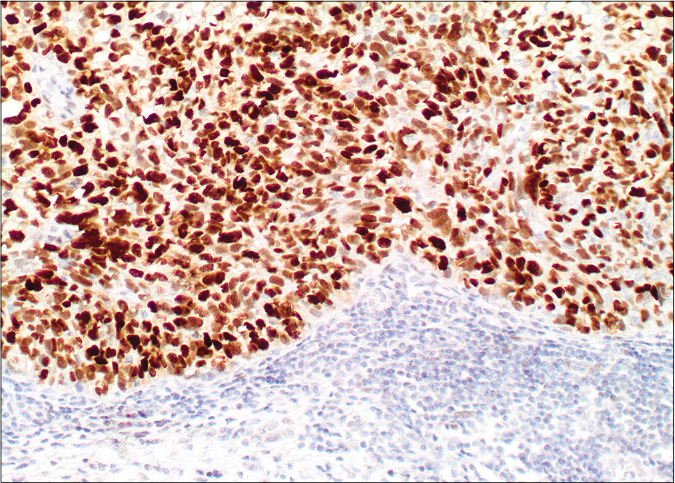
- Diffuse nuclear labelling by androgen receptor antibody (×20)
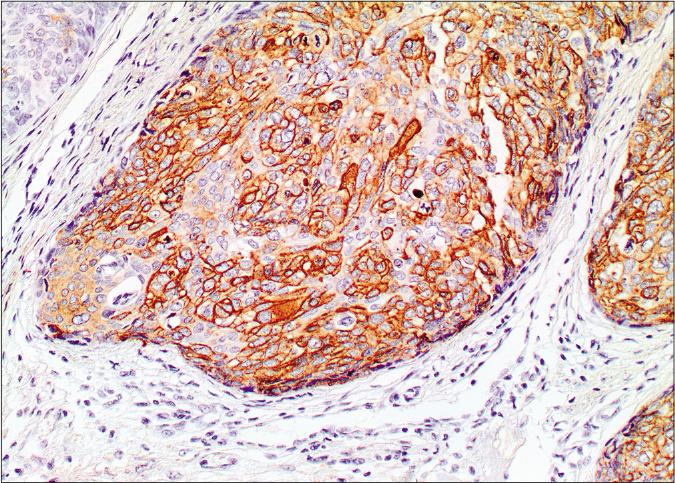
- Membranous and cytoplasmic staining by epithelial membrane antigen (×20)
Human papillomavirus status was evaluated by a fluorescently labeled polymerase chain reaction followed by a chip for human papillomavirus markers [Figure 3a]. The quantification of the associated human papillomavirus types was done by the euroarray technique using standard protocols as recommended by the manufacturer (Affymetrix, Santa Clara). In the first step, sections of the viral oncogenes E6 and E7 of the human papillomavirus present in the sample were amplified by polymerase chain reaction using a multiplex primer system and at the same time, labelled with a fluorescent dye. In a second step, the products were detected using an oligonucleotide array whereby the specific binding of a fluorescently labelled polymerase chain reaction product to its corresponding oligonucleotide probe was detected using the Euroimmunmicroarray scanner. The analysis was done for the 30 high and low risk human papillomavirus subtypes (EUROArrayHPV detection kit, EUROIMMUN AG, Germany). Human papillomavirus 66 strain was detected in both lesions [Figure 3b].
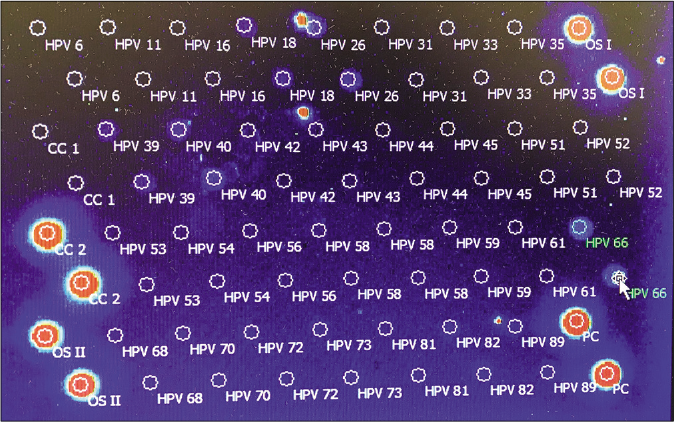
- The EUROArrayhuman papillomavirus is composed of a total of 72 spots placed in a certain arrangement
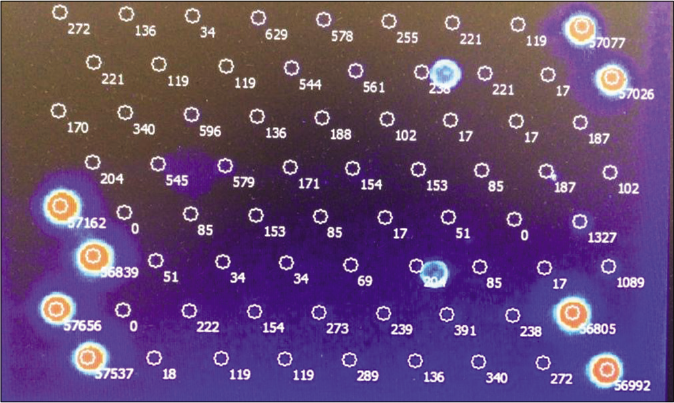
- Scanned microarray showing the signal of each probe. Only human papillomavirus 66 displays a positive signal intensity. PC: DNA positive control. CC-I and CC-II: Cross contamination control: Only one of the two probes should show a signal. If both probes show a signal, this indicates cross contamination between neighboring slide fields. OS I and OS II: Orientation spots for the automatic detection of array positions using EURO Array Scan
Human papillomavirus has emerged as a key etiologic driver in a variety of clinical conditions that range from innocuous lesions to cancers.4,5 It can infect both the cutaneous and mucosal surfaces. These viruses can be categorized into high-risk and low-risk human papillomavirus types, based on their association with cancerous and precursor lesions. The high-risk human papillomavirus types are types 16, 18, 31, 33, 34, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 and 70.4,5
In sebaceous neoplasms, mainly in carcinomas, there is a dichotomization of the pathogenesis. There are sebaceous carcinomas which harb or TP53 or RB1 mutations and a group of carcinomas which are driven by human papillomavirus infections.4 Two main viral proteins, E6 and E7, are largely responsible for the tumor carcinogenesis when incorporated into human DNA. These proteins work by inhibiting the two tumor suppressor proteins; TP53 and Rb, respectively.5 This can explain the development of sebaceous carcinomas in cases that are negative for the driving mutations (p53 and Rb) but positive for human papillomavirus infections.4,5
The segregation of sebaceous carcinomas is important since the human papillomavirus-associated tumors that are more likely to occur in younger individuals, are less aggressive and rarely display worrisome histological phenotypes including higher-grade nuclear features, warranting less aggressive postoperative surveillance.4 Furthermore, the identification of a link connecting human papillomavirus infections and the abrogation of TP53/ Rb function can also explain the reported predisposition of immunosuppressed patients to develop ocular adnexal sebaceous carcinomas. These carcinomas are more likely to occur in solid organ-transplant and HIV infected patients.4
The reported incidence of high-risk human papillomavirus in periocular sebaceous carcinoma ranges from 0% to 57%.5 In contrast, human papillomavirus profiling studies in benign sebaceous tumors are lacking. Interestingly, sebaceomas can rarely portray proliferation of infundibular keratinocytes with hypergranulosis mimicking verruca or seborrheic keratosis.6 No human papillomavirus studies have been done on this variant. Moreover, there is a report of a sebaceous gland hyperplasia accompanied with a papilloma positive for the high-risk human papillomavirus-51.7
In conclusion, our report highlights that the usage of immunomodulators such as ruxolitinib continues to expand the clinicopathological spectrum of human papillomavirus-associated neoplasms.8 One limitation is that polymerase chain reaction is a highly sensitive technique of DNA detection; hence, the presence of human papillomavirus DNA does not necessarily correlate with transcriptional activity.5 This requires more specific sequencing techniques such as in situ hybridization.4 Our future perspectives include performing these techniques on larger cohorts of patients with sebaceous neoplasms to fully understand the molecular landscape of such rare tumors. These studies may help clarify the role of human papillomavirus in sebaceous tumor carcinogenesis and possibly identify targeted or immune-based therapies such as human papillomavirus vaccination in the predisposed individuals to further improve patient outcomes.4
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Aggressive skin cancers occurring in patients treated with the Janus kinase inhibitor ruxolitinib. J Drugs Dermatol. 2017;16:508-11.
- [Google Scholar]
- Aggressive Merkel cell carcinoma after Janus kinase inhibitor ruxolitinib for polycythemia vera. In Vivo. 2019;33:1667-9.
- [CrossRef] [PubMed] [Google Scholar]
- Eruptive squamous cell carcinomas with keratoacanthoma like features in a patient treated with ruxolitinib. Br J Dermatol. 2015;173:1098-9.
- [CrossRef] [PubMed] [Google Scholar]
- Distinct biological types of ocular adnexal sebaceous carcinoma: HPV driven and virus negative tumors arise through nonoverlapping molecular genetic alterations. Clin Cancer Res. 2019;25:1280-90.
- [CrossRef] [PubMed] [Google Scholar]
- p16 expression is not a surrogate marker for high risk human papillomavirus infection in periocular sebaceous carcinoma. Am J Ophthalmol. 2016;170:168-75.
- [CrossRef] [PubMed] [Google Scholar]
- Sebaceoma and related neoplasms with sebaceous differentiation: A clinicopathologic study of 30 cases. Am J Dermatopathol. 2002;24:294-304.
- [CrossRef] [PubMed] [Google Scholar]
- Papilloma and sebaceous gland hyperplasia of the lacrimal caruncle: A case report. Int Med Case Rep J. 2018;11:91-5.
- [CrossRef] [PubMed] [Google Scholar]
- The evolving landscape of HPV related neoplasia in the head and neck. Hum Pathol. 2019;94:29-39.
- [CrossRef] [PubMed] [Google Scholar]





