Translate this page into:
Histopathologic study of morphea
Corresponding author: Dr. Inchara Yeliur Kalegowda, Department of Pathology, St. John’s Medical College, Bengaluru, India. inchara.yk@stjohns.in
-
Received: ,
Accepted: ,
How to cite this article: Tharappel DCP, Ballal S, Kalegowda IY. Histopathologic study of morphea. Indian J Dermatol Venereol Leprol. 2025;91:257-60. doi: 10.25259/IJDVL_316_2024
Dear Editor,
Morphea (localised scleroderma) is a rare inflammatory fibrosing disease of the skin.1,2 Patterns of sclerosis and degree of inflammation are known to have clinical implications such as increased functional limitations.2 Histopathology of morphea has received little attention in India.3,4
This retrospective study was performed at a tertiary care centre. Cases diagnosed based on clinical suspicion and histopathologic confirmation from 2012 to 2020 were included. In this study, lichen sclerosus (LS) was excluded as it was not considered a variant of morphea. Skin biopsies were studied for histopathologic features. Patterns of arrangement of lymphocytes were categorised as ‘scattered interstitial’, ‘Indian file’ and ‘lymphoid aggregate’.
There were 101 skin biopsies from 78 patients (17 paediatric cases). Age ranged from 6 to 94 years. Female preponderance was noted (n = 46, 59%). The lesions were seen on the head and neck (n = 13) [Figure 1a-b], trunk (n = 35) and extremities (n = 78, 77%). The most common clinical subtype was the plaque type (n = 32, 43%).
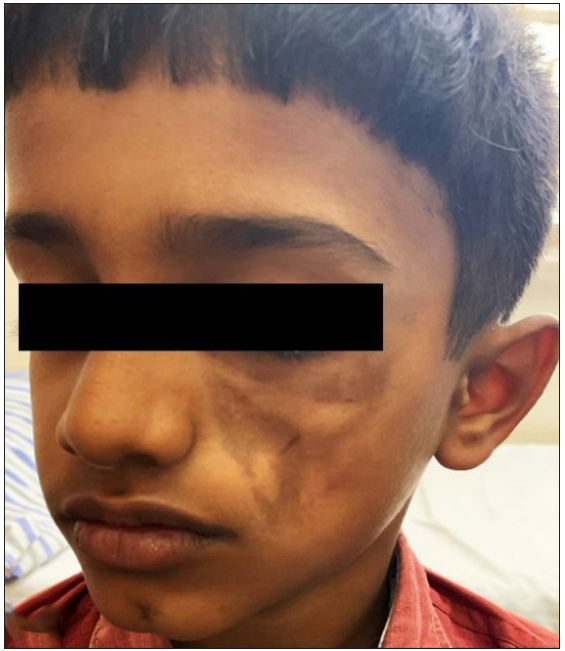
- Plaque-type morphea affecting the face in a child.
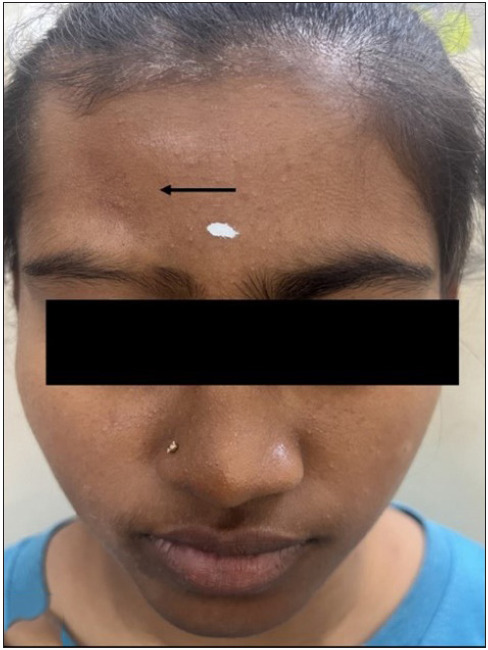
- Linear morphea (black arrow) affecting the frontoparietal region.
Dermal sclerosis, which was present in all cases, was either top-heavy [Figure 2a], bottom-heavy [Figure 2b], full thickness [Figure 2c] or rarely patchy or focal. The histopathologic features are depicted in Tables 1 and 2. All the cases with top-heavy sclerosis also showed involvement of the mid-dermis in addition to the papillary dermis. A few features were distinctly absent in certain groups.
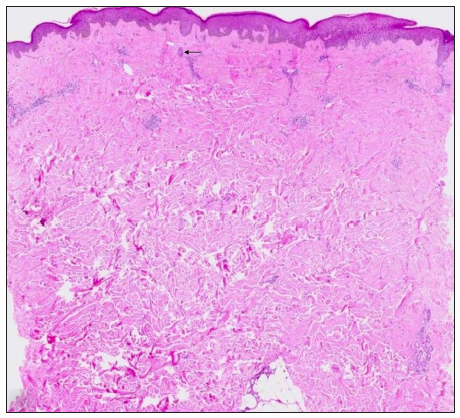
- Top-heavy sclerosis with follicular remnants seen as columnar, basaloid structures (black arrow). (Haematoxylin and eosin,10x)
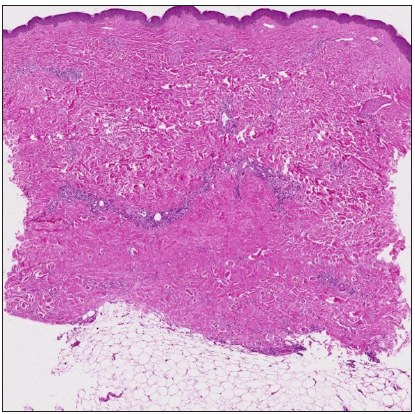
- Bottom-heavy sclerosis. (Haematoxylin and eosin, 20x)
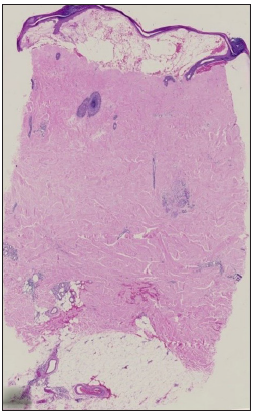
- Full-thickness sclerosis, ‘square biopsy’ sign and ‘line’ sign. (Haematoxylin and eosin, 10x)
|
Histopathological features of biopsies (n = 101) (n, %) |
Micro-anatomical location of dermal sclerosis (n = 101) | |||
|---|---|---|---|---|
|
Top heavy (n = 6) |
Bottom heavy (n = 17) |
Full thickness (n = 70) |
Patchy/focal (n = 8) |
|
|
‘Square biopsy’ sign (n = 42, 42%) (square-/rectangle-shaped biopsy) |
1 | 3 | 38 | 0 |
|
‘Line’ sign (n = 45, 45%) (straight line at dermis-subcutis interface) |
0 | 4 | 40 | 1 |
|
‘Cookie cutter’ sign (n = 40, 40%) (parallel lateral edges of biopsy) |
2 | 4 | 33 | 1 |
| High eccrine glands (n = 51, 50%) | 2 | 8 | 40 | 1 |
| Epidermal atrophy (n = 18, 18%) | 1 | 0 | 15 | 2 |
| Entrapped fat in dermis (n = 49, 49%) | 0 | 8 | 40 | 1 |
|
Lichen sclerosus-like changes (n = 15, 15%) (papillary dermal homogenisation of collagen, focal basal vacuolar alteration of epidermis and dermal melanophages) |
2 | 0 | 12 | 1 |
| Diminished adnexa (n = 76, 75%) | 5 | 9 | 58 | 4 |
| Inflammation (n = 75, 74%) | ||||
|
‘Scattered interstitial’ (n = 48, 64%) (scattered lymphocytes between collagen bundles) |
2 | 2 | 43 | 1 |
|
‘Indian file’ (n = 19, 25%) (linear arrangement of lymphocytes) |
1 | 2 | 16 | 0 |
|
Lymphoid aggregate (n = 22, 29%) (circumscribed arrangement of lymphocytes) |
0 | 2 | 20 | 0 |
| Perineural inflammation (n = 20, 27%) | 0 | 2 | 17 | 1 |
| Inflammation (n = 75) | n (%) |
|---|---|
| Location | |
| Superficial dermis | 52 (69%) |
| Mid-dermis | 27 (36%) |
| Deep dermis | 34 (45%) |
| Spill of inflammation into subcutis | 12 (16%) |
| Intensity | |
| Mild | 34 (45%) |
| Moderate | 38 (51%) |
| Severe | 24 (32%) |
| Distribution | |
| Perivascular | 56 (75%) |
| Periadnexal | 10 (13%) |
| Perineural | 20 (27%) |
| Junction of dermis and subcutis | 3 (4%) |
| Composition | |
| Lymphocytes | 75 (100%) |
| Plasma cells | 45 (60%) |
| Mixed (+eosinophils/neutrophils) | 7 (9%) |
| The pattern of arrangement of lymphocytes | |
| ‘Scattered interstitial’ | 48 (64%) |
| ‘Indian file’ | 19 (24%) |
| ‘Lymphoid aggregate’ | 22 (29%) |
The demographics and clinical types were concordant with the literature. Extremities were commonly affected and most cases showed full-thickness sclerosis, contrary to the cases seen in literature.2 The presence of top-heavy sclerosis supports the concept that morphea may be limited to the superficial reticular dermis, known as superficial morphea.2 Diminished skin adnexal structures were consistent findings.5 Remnant follicles, appearing as columnar basaloid structures were more frequently sighted than remnant sebaceous and eccrine glands.
The ‘line’ sign was never seen in the top-heavy pattern of sclerosis. This is explainable because the ‘line’ sign is seen only when the sclerosis involves the dermal-subcutis interface and depends on the presence of subcutis and the depth of the biopsy. However, if the subcutis is replaced by collagen, even a deep biopsy may not show apparent subcutis. The other named signs were seen in fewer cases when compared to literature.3
As per literature, the incidence of overlying lichen sclerosus-like changes in morphea ranges from 12% to 24%.5 We observed this in a few cases which showed either a top-heavy or full-thickness pattern of dermal sclerosis, suggesting that this probably occurs when the upper portion of the dermis is involved. The link between these two entities is controversial.
The pattern of arrangement of lymphocytes in morphea was interesting. A ‘scattered interstitial’ pattern [Figure 3a] predominated even in cases where sclerosis was patchy/focal. Sometimes, they formed linear arrays, the ‘Indian file’ pattern [Figure 3b]. It is said that detecting interstitial dermal infiltrate on a histopathology section requires more alertness as compared to detecting perivascular and periadnexal infiltrates as per mechanisms of visual perception.6 Lymphocytes in linear arrays have been acknowledged in occasional case reports but only in passing. An Indian study describes this kind of pattern as ‘lymphocyte peppered sclerotic collagen’ which was observed mainly in LS (80% of cases) followed by morphea (50% of cases).4 This pattern can serve as a clue to identify early lesions on histopathology wherein sclerosis has not yet fully set in, thus helping in initiating early therapy.
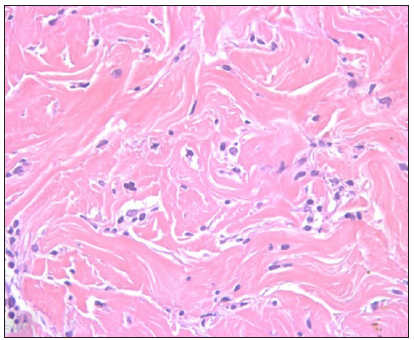
- ‘Scattered interstitial’ pattern of lymphocytes amidst the sclerotic collagen. (Haematoxylin and eosin, 400x)
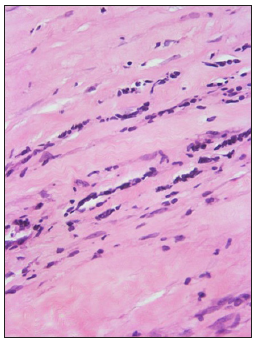
- Lymphocytes in linear arrays, the ‘Indian file’ pattern. (Haematoxylin and eosin, 400x)
Another pattern observed was the lymphoid aggregate formation [Figure 3c]. This may be seen in late sclerotic morphea, at the dermis-subcutis interface.
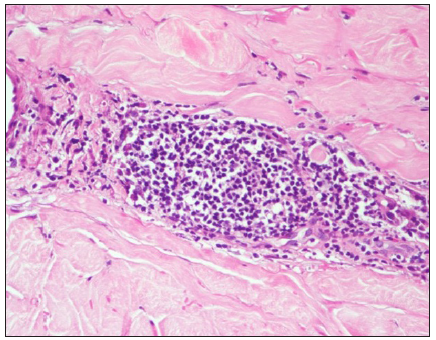
- ‘Lymphoid aggregate’ formation. (Haematoxylin and eosin, 400x)
Perineural inflammation observed in this study has been reported by others in 52%–84% of cases.5,7 While Hansen’s disease strikes the mind commonly in our practice, one should be aware of other entities like morphea when we see this feature.7
Plasma cells were the second most common inflammatory cell in a study,2 similar to ours. Their presence is consonant with current theories of the mechanism of fibrosis, hinting at a T helper cell 2-dominated immune response.2
Limitations
The limitation of this study was that it was a single-centre retrospective study.
Conclusion
Histopathologic features in morphea are variable. Dermal sclerosis and diminished/absent adnexal structures are the most consistent histologic parameters. Named histologic signs that are widely patronised in literature are not consistently present and largely depend on the microanatomical location of sclerosis. The most common inflammatory pattern is the ‘scattered interstitial’ pattern of lymphocytes. Attention to pointers such as patterns of arrangement of inflammatory cells, perineural inflammation and the presence of plasma cells can aid diagnosis in difficult cases or when sclerosis is partial/limited. Knowledge of the depth of involvement by sclerosis and inflammatory activity may provide insights aiding in the clinical evaluation of patients in future studies.
Ethical approval
The research/study was approved by the Institutional Review Board at St. John’s Medical College, number 265/2018, dated 10.10.2018.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Connective tissue diseases. In: Ramam M, Khandpur S, Bhari N, Gupta V, eds. IADVL Textbook of dermatopathology. New Delhi: Jaypee Brothers Medical Publishers; 2023. p. :189-99.
- [Google Scholar]
- Histopathological changes in morphea and their clinical correlates: Results from the morphea in adults and children cohort V. J Am Acad Dermatol. 2017;76:1124-30.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Histopathology of morphea: Sensitivity of various named signs, a retrospective study. Indian J Pathol Microbiol. 2020;63:600-3.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphocyte-peppered sclerotic collagen: An additional histological clue in lichen sclerosus, morphea, and systemic sclerosis. Am J Dermatopathol. 2021;43:935-8.
- [CrossRef] [PubMed] [Google Scholar]
- Histopathologic spectrum of morphea. Am J Dermatopathol. 2021;43:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- Interstitial dermal infiltrate: An intriguing pattern. Diagn Histopathol. 2021;27:13-25.
- [Google Scholar]
- Perineural inflammation in morphea (localized scleroderma): Systematic characterization of a poorly recognized but potentially useful histopathological feature. J Cutan Pathol. 2014;41:28-35.
- [CrossRef] [PubMed] [Google Scholar]





