Translate this page into:
Histopathological characteristics of post kala-azar dermal leishmaniasis: A series of 88 patients
2 Department of Dermatology, Safdarjung Hospital, New Delhi, India
3 Department of Dermato-Venereology, All India Institute of Medical Sciences, New Delhi, India
Correspondence Address:
Avninder Singh
National Institute of Pathology ICMR, Safdarjung Hospital Campus, New Delhi - 110 029
India
| How to cite this article: Singh A, Ramesh V, Ramam M. Histopathological characteristics of post kala-azar dermal leishmaniasis: A series of 88 patients. Indian J Dermatol Venereol Leprol 2015;81:29-34 |
Abstract
Background: Post kala azar dermal leishmaniasis (PKDL) is a sequel to visceral leishmaniasis or kala azar seen predominantly in the Indian subcontinent and Africa. Histopathological descriptions of the condition are limited. Methods: Biopsies of 88 skin and 16 mucosal lesions were evaluated for histopathological findings on formalin-fixed, paraffin-embedded tissues. Results: There were 71 (80.7%) males and 17 (19.3%) females with a mean age of 24.8 and 28.5 years, respectively. A past history of kala azar was present in 64 (72.7%) patients and post kala azar dermal leishmaniasis developed a mean of 6.2 years after visceral leishmaniasis. Of the biopsies studied, the clinical lesions were macular in 14 (15.9%), papulo-nodular in 32 (36.3%) and showed both macules and papulo-nodules in 42 (47.8%). Follicular plugging was a common epidermal finding. A clear Grenz zone was frequently noted. The dermal infiltrates were arranged mainly in three patterns: superficial perivascular infiltrates in 16 (18.1%), perivascular and perifollicular infiltrates in 24 (27.3%) and diffuse infiltrates in 41 (46.6%) biopsies. Leishman-Donovan (LD) bodies were noted in 13 (44.9%) of 69 cases on slit-skin smear and in 25 (28.4%) of 88 biopsies. In 16 patients, where both skin and mucosal biopsies were available, LD bodies were identified in 10 (62.5%) mucosal biopsies as compared to 3 (18.7%) skin biopsies. Limitations: The retrospective nature of the study and the lack of controls were limitations. Conclusion: The various histomorphological patterns of post kala azar dermal leishmaniasis are a useful clue to the diagnosis even when LD bodies have not been detected. This study also suggests that LD bodies are more frequently seen in mucosal biopsies in comparison to cutaneous biopsies.Introduction
Post-kala-azar dermal leishmanasis (PKDL) is an uncommon sequel seen in patients with a previous attack of visceral leishmaniasis or kala azar and was first described by Brahmachari, in 1922. [1] In 15-20% of the cases there is no previous history of visceral leishmaniasis but these patients invariably hailed from the kala azar endemic region. [2]
Post kala azar dermal leishmaniasis is clinically characterized by hypopigmented macules and erythematous papules, plaques and nodules. We present the histopathological findings noted in 88 biopsies of patients attending our hospital.
Methods
This is a retrospective study in which we reviewed the records of patients who attended the outpatient clinic of the department of dermatology, Safdarjung Hospital, New Delhi with clinical lesions suggestive of PKDL between 2004 and 2011. The clinical criteria for diagnosing PKDL were (a) history of living in kala azar endemic region, (b) positive rk39 immunochromatographic strip test against promastigote/amastigote antigen (c) response to treatment with either sodium stibogluconate, miltefosine or amphotericin B, (d) exclusion of other infective dermatoses notably leprosy because of clinical hypopigmentation and secondary syphilis due to presence of plasma cells in the infiltrate. There were 92 patients with a clinical diagnosis of PKDL over a period of 7 years (2004-2011). All 92 patients hailed from the state of Bihar which is endemic for kala azar. Four patients were excluded from the study because three were diagnosed as leprosy and one as vitiligo after histopathologic examination. Patients were asked about previous history of kala azar, general clinical examination was done and their age, gender, type and distribution of the cutaneous lesions were recorded. Giemsa-stained slit skin smears were performed and results were available for 69 patients. Punch biopsies were taken in all cases. All the authors reviewed hematoxylin-eosin stained slides of each case and a consensus about the histopathological findings was reached. Fite-Faraco stain was done to exclude acid-fast bacilli in cases where foamy histiocytes or perineural infiltrates were present.
Results
The clinical data is shown in [Table - 1]. The male to female ratio was 4:1 with the mean age at the time of diagnosis in female patients being greater as compared to males. Sixty four (72.7%) of 88 patients had a previous history of kala azar. The interval between the attack of kala azar and the development of cutaneous lesions of post kala azar dermal leishmanisis ranged from 1 year to 20 years with a mean duration of 6.2 years. The clinical lesions were divided into three categories; purely macular lesions were seen in 14 (15.9%) [[Figure - 1]a], polymorphic (both macules and papulo-nodular) lesions were seen concurrently in 42 (47.8%) [[Figure - 1]b] and the remaining 32 (36.3%) were papulo-nodular lesions. Sixteen (18.2%) patients additionally had mucosal involvement in lip [[Figure - 1]b], tongue [[Figure - 1]c] and glans penis [[Figure - 1]d].
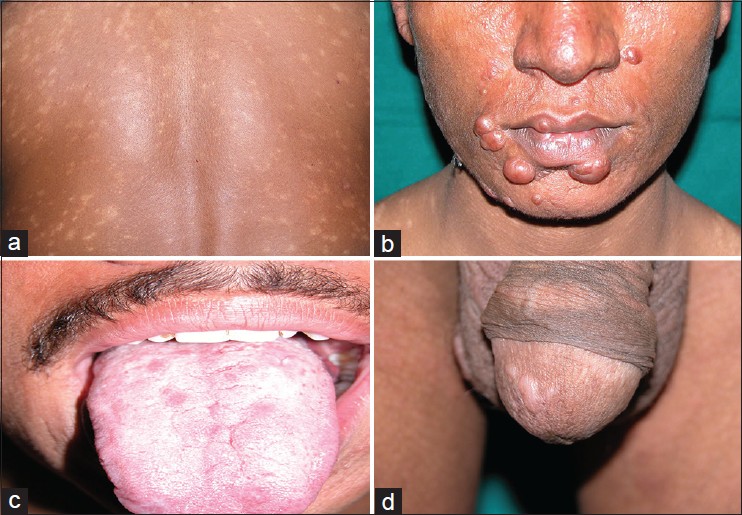 |
| Figure 1: PKDL showing (a) generalized hypopigmented macules, (b) papulonodules on face and lip, (c) papules on tongue, (d) papules on glans penis |
Slit smear examination identified intracellular amastigotes in 31 (44.9%) of 69 patients.
Histopathological findings summarized in [Table - 2] show that normal epidermal thickness with follicular plugging and the presence of a clear Grenz zone between the epidermis the dermal infiltrate were the most common findings. The dermal inflammatory infiltrate was arranged predominantly in three patterns; superficial perivascular inflammation was seen in biopsies from macules that showed a mild to moderate infiltrate of plasma cells and lympho-histiocytes. Biopsies from papules and plaques showed follicular plugging and a dense dermal perivascular and perifollicular infiltrate in the upper half of the dermis separated from epidermis by a band of normal dermal collagen [Figure - 2]. The third pattern showed diffuse dermal infiltrate in biopsies from nodules. The inflammatory cells consisted of a mixture of histiocytes, lymphocytes and plasma cells. The histiocytes were noted to have foamy cytoplasm in 30 (34%) cases, an appearance that suggested a diagnosis of leprosy. Fite-Faraco staining for acid fast bacilli was negative in all cases. LD bodies were detected on biopsy in 25 (28.4%) of 88 cases.
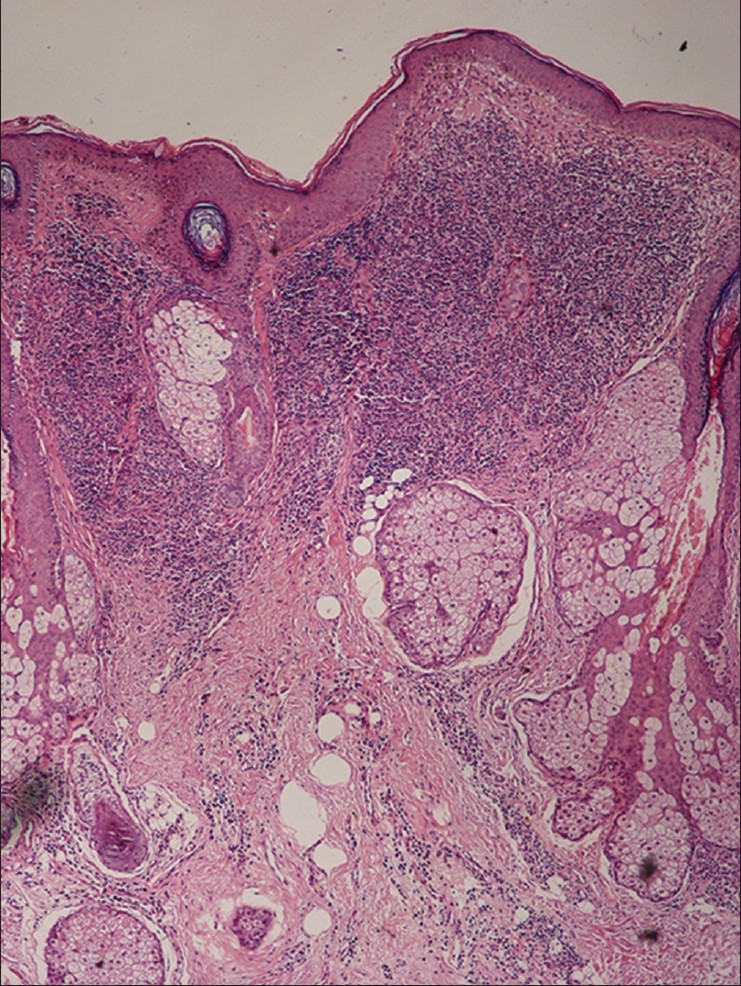 |
| Figure 2: Biopsy of a papule showing follicular plugging and a dense lymphohistiocytic infiltrate in the top half of dermis (H and E, x100) |
All 14 cases of pure macular post kala azar dermal leishmaniasis showed a sparse superficial perivascular infiltrate consisting of lymphocytes, histiocytes and some plasma cells. The overlying epidermis was unremarkable. No amastigotes were identified in these biopsies.
Biopsy from the papule/plaque showed a normal to hyperplastic epidermis with follicular plugging in 57 biopsies while a clear Grenz zone was noted in 61 biopsies. Interface changes at the dermo-epidermal junction were seen in five patients and three of these cases also showed exocytosis. Papillary dermal edema was noted in 11 biopsies. The dermis showed a dense, superficial and deep perivascular infiltrate consisting of histiocytes, lymphocytes and plasma cells. In two cases, a curvilinear pattern of inflammation around the neurovascular bundles reminiscent of leprosy was seen. Four cases showed a granulomatous infiltrate with nodular aggregates of pale staining macrophages and epitheloid cells surrounded by lymphocytes and plasma cells; the epidermis was atrophic over the infiltrate in these cases. One biopsy showed an atrophic epidermis, Grenz zone of normal collagen and a diffuse dermal infiltration by foamy histiocytes mimicking lepromatous leprosy [Figure - 3]. The histiocytes showed numerous intracytoplasmic amastigotes [Figure - 4]. Two biopsies showed a perineural infiltrate of lymphomononuclear cells while another two showed a predominantly peri-eccrine infiltrate of lymphocytes, histiocytes and plasma cells [Figure - 5]. Six biopsies showed perivascular sclerosis in dermal vessels [Figure - 6] and one showed calcification mimicking a Schaumann body.
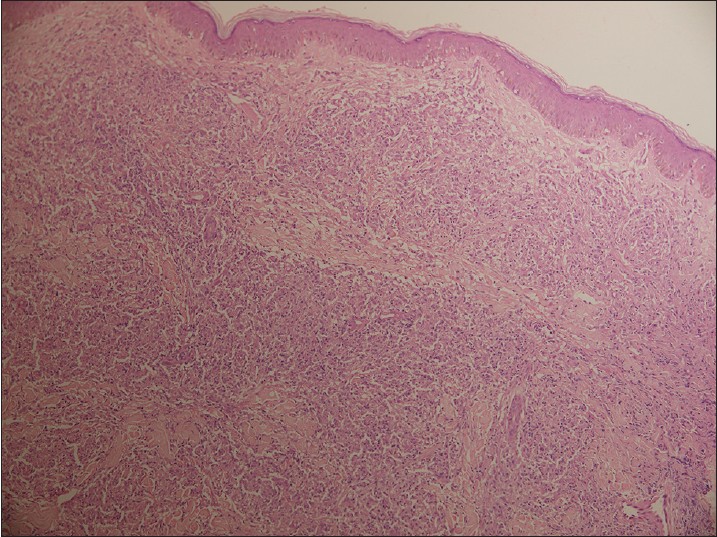 |
| Figure 3: Biopsy from a nodule showing an atrophic epidermis, subepidermal grenz zone and a dermal infiltrate of pale staining macrophages and lymphocytes mimicking lepromatous leprosy (H and E, x100) |
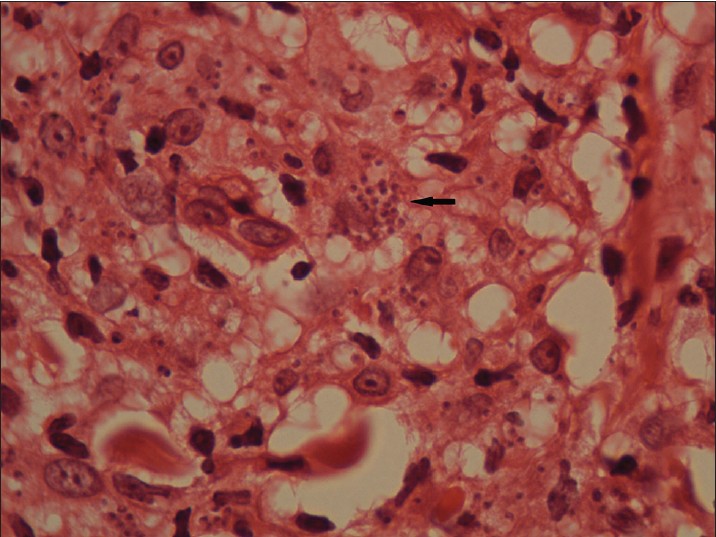 |
| Figure 4: Numerous intracytoplasmic amastigotes (arrows) in the nodule pictured in Fig 3 (H and E, x1000) |
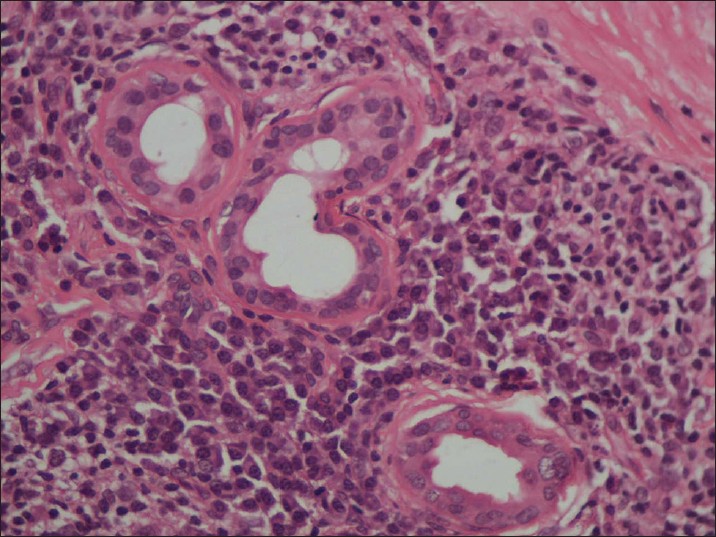 |
| Figure 5: The perieccrine infiltrate shows numerous plasma cells (H and E, x200) |
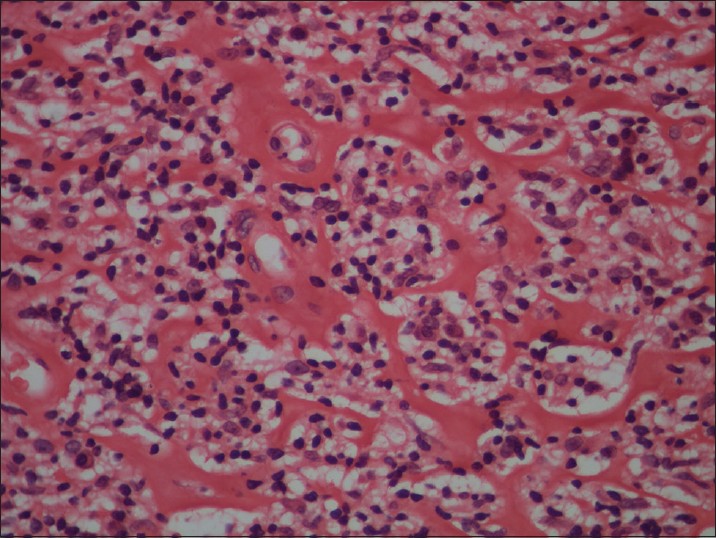 |
| Figure 6: Perivascular sclerosis in dermal capillaries (H and E, x200) |
Sixteen (18.2%) biopsies were obtained from mucosal lesions, where lip (11 biopsies) was the most common site of predilection followed by glans penis (8 biopsies) and tongue (5 biopsies).
We found no LD bodies in biopsies from macular lesions. LD bodies were noted in 16 (38.1%) of 42 biopsies from polymorphic lesions and 9 (28.1%) of 32 biopsies from papulonodular lesions. LD bodies were present in 10 (62.5%%) of 16 mucosal biopsies compared to 3 (18.8%) of 16 skin biopsies, in cases where both skin and mucosal biopsies were available for comparison in the same patient.
Discussion
Post kala azar dermal leishmaniasis has been reported from the Indian subcontinent and Africa, but there are important differences in the manifestations of disease in these geographical regions. In India, post kala azar dermal leishmaniasis follows visceral leishmaniasis in 5%-10% of patients [2] compared to 50% in Africa. [3] The interval between occurrence of kala azar and PKDL ranges from 1 to 20 years (mean 6.2 years) in Indian PKDL unlike African PKDL that usually develops within a few months to an year after visceral leishmaniasis. [4] Indian PKDL differs histologically from African PKDL by being less granulomatous and with more plasma cells in the infiltrate. Finally, African disease is usually self-limited and resolves in 6-12 months while the Indian disease persists indefinitely without treatment and is difficult to treat. [5] These features indicate that Indian PKDL is probably a distinct disease.
Hypopigmented macules are the most common lesions in PKDL, usually occurring in combination with other lesions. A purely macular presentation has been observed in about 10% of Indian PKDL patients in Indian reports [6],[7] In a series of 14 cases of purely macular PKDL, Ramesh and Singh [8] described three distinct clinical patterns, localized, generalized and extensive. Biopsy from macular lesions reveal a sparse inflammatory infiltrate of lymphocytes, histiocytes and a few plasma cells predominantly around the vessels of the superficial vascular plexus. Parasites are absent to scanty in the macular variant. When sparse, they may be difficult to recognize and may occasionally be confused with karyorrhectic nuclear debris of lymphocytes. In the absence of LD bodies, plasma cells in the inflammatory infiltrate are a clue to the diagnosis of PKDL. However, the diagnosis is usually based on history, clinical examination, exclusion of other diseases and response to therapy. Clinically, important differential diagnoses are indeterminate and macular variants of leprosy. Unlike macular PKDL where the infiltrate is superficial, both indeterminate and macular lesions of leprosy show, in addition, inflammatory infiltrate centered around the neurovascular plexus in the lower dermis. [8]
The commonest clinical presentation is a combination of macules, papules, and nodules. In papules, plaques and nodules, the epidermis may be normal, hyperplastic or atrophic. In addition, there may be interface changes characterized by vacuolar degeneration of the basal cells and infiltration of basal cell layer by lymphocytes, seen in 5 biopsies in our study. One worker has attributed the hypopigmentation of post kala azar dermal leishmaniasis to this lymphocytic infiltration of the basal layer, acting by damage to basal keratinocytes that are in intimate contact with melanocytes. [9]
Papules and plaques usually showed a moderate to dense inflammatory cell infiltrate, though rarely, the infiltrate was sparse. Lymphocytes were the predominant cells when the infiltrate was moderate in intensity but as the density of infiltrate increased, histiocytes and plasma cells were found in greater numbers.
Nodules showed a dense, diffuse dermal inflammatory infiltrate consisting of histiocytes and plasma cells in large numbers. The epidermis overlying the infiltrate was stretched by the bulk of inflammatory cells. In contrast to lepromatous leprosy where the peripheral limits of the infiltrate are infiltrative, nodular post kala azar dermal leishmaniasis has a fairly sharp margin. Compact epithelioid cell granulomas were seen in a minority (4 (4.5%)) of 88 biopsies, all from nodules. As in a previous report, we did not find granulomas in other kinds of lesions. [10] One biopsy from a nodule showed a diffuse dermal infiltrate of foamy macrophages mimicking lepromatous leprosy. However, these macrophages were loaded with LD bodies making the diagnosis obvious. Other less common observations were perineural infiltrates in two cases as noted by previous authors [11],[12] that compounded the diagnostic difficulties between leprosy and PKDL. Sclerosis around the dermal vessel walls was seen in six cases, an observation also reported earlier. [10]
In a previous study of 26 nodular lesions, Singh et al.,[10] reported that the epidermis was atrophic and the dermal adnexae were either caught up in the infiltrate or were displaced downwards.
We found amastigotes in 25 (28.4%) of 88 biopsies, lower than the 13 (44.9%) of 69 cases detected by slit skin smear examination in the same patients. This is similar to the detection rate of 10 (25%) of 40 cases in the study by Rathi et al. [13] A prolonged search is necessary as parasites are often sparse. LD bodies, if scanty, are best found just beneath the epidermis. [7] Sometimes, nuclear debris may mimic LD bodies but it has been our observation that nuclear detritus has an intense hematoxyphilic color similar to that of lymphocyte nuclei whereas LD bodies are a shade paler. When LD bodies are numerous, they can be confused with histoplasmosis but the latter can be identified by stains for fungi, such as PAS and silver methanamine.
In a study of 50 PKDL patients, Beena et al. [14] found LD bodies in 25 (50%) biopsies on hematoxylin and eosin stained sections. Special stains such as Giemsa stain or iron hematoxylin did not have any significant advantage in staining the amastigotes. They found that immunohistochemical (IHC) detection of LD bodies by using G2D10, a mouse monoclonal antibody raised against a promastigote membrane antigen of L. gerbelli improved the detection rate to 80%. Similarly, a study in 30 Sudanese PKDL patients found that a detection rate of LD bodies of 17% on hematoxylin-eosin stain increased to 88% when using a monoclonal antibody (D2)(2E5-A8) specific to L. donovani. [15],[16]
As in the study by Rathi et al., [13] we found LD bodies only in plaques and nodules. Like them, we were unable to find amastigotes in any biopsies from macules of post kala azar dermal leishmaniasis. Beena at al [14] were also unable to demonstrate any LD bodies on hematoxylin and eosin stained slides of macular PKDL. However, they found LD bodies in 4 of 11 cases using the G2D10 immunostain.
An affinity of lesions for genital skin and mucosa has been previously noted [17] We studied 16 mucosal biopsies from the inner lip, tongue, glans penis and palate. LD bodies were more frequently demonstrable in 10 (62.5%) of 16 mucosal biopsies, a higher frequency than the 3 (18.8%) of 16 noted in cutaneous biopsies of the same patients. We were unable to find any previous reports of this observation. When a patient presents with both cutaneous and mucosal lesions, a mucosal biopsy may be preferred as it is more likely to yield a parasitological diagnosis.
| 1. |
Brahmachari UN. A new form of cutaneous leishmaniasis-dermal leishmanoid. Indian Med Gaz 1927; 11-2.
[Google Scholar]
|
| 2. |
Mukherjee A, Ramesh V, Mishra RS. Post kala azar dermal leishmaniasis: A light and electron microscopic study of 18 cases. J Cutan Pathol 1993; 20:320-5.
[Google Scholar]
|
| 3. |
Musa AM, Khaleel EAG, Raheem MA, et al. The natural history of Sudanese post kala azar dermal leishmaniasis: Clinical, immunological and prognostic features. Ann Trop Med Parasitol 2002; 96:765-72.
[Google Scholar]
|
| 4. |
Zijlstra EE, Musa AM, Khalil EAG, El-Hassan IM, El-Hassan AM. The Lancet Infect Dis 2003; 3:88-97.
[Google Scholar]
|
| 5. |
El Hassan, Ghalib HW, Zijlstra EE, et al. Post kala azar dermal leishmaniasis in the Sudan-clinical features pathology and treatment. Trans R Soc Trop Med Hyg 1992: 86: 245-8.
[Google Scholar]
|
| 6. |
Ramesh V, Misra RS, Saxena U, Mukherjee A. Post kala azar dermal leishmaniasis: A clinical and therapeutic study. Int J Dermatol 1993:32: 27-75.
[Google Scholar]
|
| 7. |
Girgla HS, Marsden RS, Singh GM, Ryan TJ. Post kala azar dermal leishmaniasis. Br J Dermatol 1977; 97: 307-9.
[Google Scholar]
|
| 8. |
Ramesh V, Singh N. A clinical and histopathological study of macular type of post kala azar dermal leishmaniasis.Trop Doc 1999; 29: 205-9.
[Google Scholar]
|
| 9. |
Ismail A. Immune responses and immunopathology of post kala azar dermal leishmaniasis (PKDL) (PhD thesis) University of Copenhagen, Denmark, 1999.
[Google Scholar]
|
| 10. |
Singh N, Ramesh V, Arora VK, Bhatia A, Kubba A, Ramam M. Nodular post kala azar dermal leishmaniasis: A distinct histopathological entity. J Cutan Pathol 1998; 25: 95-9.
[Google Scholar]
|
| 11. |
Khandpur S, Ramam M, Sharma VK, Salotra P, Singh MK, Malhotra A. Nerve involvement in Indian post kala azar dermal leishmaniasis. Acta Derm Venereol 2003; 84: 245-6.
[Google Scholar]
|
| 12. |
El Hassan AM, Ali MS, Zijlstra EE, Eltoum IA, Ghalib HW, Ahmed HMA. Post kala azar dermal leishmaniasis in the Sudan: Peripheral neural involvement. Int J Dermatol 1992; 31:400-3.
[Google Scholar]
|
| 13. |
Rathi SK, Pandhi RK, Chopra P, Khanna N. Post kala azar dermal leishmaniasis. Indian J Dermatol Venereol Leprol 2005; 71:250-3.
[Google Scholar]
|
| 14. |
Beena KR, Ramesh V, Mukherjee A. Identification of parasite antigen, correlation of parasite density and inflammation in skin lesions of post kala azar dermal leishmaniasis. J Cutan Pathol 2003; 30: 616-20.
[Google Scholar]
|
| 15. |
Ismail A, Gadir AFA, Theander TG, Kharazami A, El Hassan AM. Pathology of post kala azar dermal leishmaniasis: A light microscopical, immunohistochemical and ultrastructural study of skin lesions and draining lymph nodes. J Cutan Pathol 2006; 33: 778-87.
[Google Scholar]
|
| 16. |
Ismail A, Kharazami A, Permin H, El Hassan AM. Detection and characterization of Leishmania in tissues of patients with post kala azar dermal leishmaniasis using a specific monoclonal antibody. Trans R Soc Trop Med Hyg 1997; 91:283-5.
[Google Scholar]
|
| 17. |
Ramesh V, Mukherjee A. Post kala azar dermal leishmaniasis. Int J Dermatol 1995; 34:85-91.
[Google Scholar]
|
Fulltext Views
6,328
PDF downloads
2,679





