Translate this page into:
History of hydroquinone
Corresponding author: Dr. Pravin Degambar Banodkar, Department of Dermatology, Skin Crest Clinic, Avanti Apartment Flat 55 5th floor Senapati Bapat Marg, Dadar West, Mumbai, 400028, Maharashtra, India. pravin_banodkar@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Banodkar PD, Banodkar KP. History of hydroquinone. Indian J Dermatol Venereol Leprol 2022;88:696-9.
Introduction
Human beings have been enthralled and prepossessed by skin colour over the centuries. It has steered political movements and has had long-lasting implications on the history, economics, and demographics of countries. Research into the origin of skin colour began in the 16th century after the invention of microscope. Investigations into the origin of pigmentation in hyper pigmentary disorders led to the histopathologic study of melanocytes. Trials for treatments of hyperpigmentation can be traced to the works of Dr.Jacobi. He had experimented on hyperpigmentary disorders by applying borax, sulphur, tincture of iodine, potassium and sodium hydroxide.1 Complications like blisters caused by irritant contact dermatitis were a common occurrence. Other chemicals commonly used were ammoniated mercury 3% in cream formulations. The efforts and clinical trials substantiate the idea that disorders of skin colour such as hyperpigmentation were a notable problem.
History of colourism and relevance to the billion-dollar skin whitening market in India
Colourism, the act of discriminating against a person due to differences in skin colour—has been used to advance and oppress individuals for centuries but has been masked by conversations of race. Many scriptures contain data on including dark-complexioned characters among the leaders, singers, and intellectuals. Names of Indian deities like Kali and Krishna translate to “black” in Sanskrit.
The foreign invasions of India—by the lighter-skinned Mughals, Portuguese and British— led to skin colour become a social stratification tool and colourism was introduced. During British colonization, they provided light-skinned Indians more job opportunities than dark-skinned Indians. The world witnessed the formation of Black and White towns to discriminate the populations.8
The use of skin-bleaching products to obtain lighter skin complexion has skyrocketed over the years. The global skin-lightening market is expected to reach $31.2 billion by 2024. Indian skin-whitening market is valued at over $200 million, accounting for 46 per cent of the facial care market and far exceeding Coca Cola and tea industries.9
The skin-colour caste system has also infiltrated the school environment. Scholars have noted that children of colour develop colour consciousness early on as a result of internalizing their families’ “colour complex” and facing increased disciplinary action in school. An increasing phenomenon seen in our daily practice is to see parents bringing in their children for skin-lightening treatments.
History of skin bleaching
The practice of skin bleaching has been traced to 200 B.C.E and has transcended through generations and civilisation from Greeks, Romans, Egyptians to the Chinese and Japanese. Exogenous ochronosis has been found in the skin layers of a mummy’s head unearthed at the Theban necropolis which is an ancient burial ground that covers a vast area on Luxor’s West Bank of the Nile River in Upper Egypt, which is currently linked to whitening products such as hydroquinone.2
Conversely, India’s skin-lightening history is relatively new. The 15th century sparked a national shift in preference for fairer skin. Some of the earliest attempts at skin-lightening included an application of turmeric and milk. Indian mothers would drink milk with saffron for a “fairer-toned” foetus.2 More drastic lotions have flooded the market over the centuries.
Hydroquinone in early years
Hydroquinone or 1,4 dihydroxybenzene was obtained by dry distillation of quinic acid by Pelletier and Caventou in 1820. By 1884, Friedrich Wohler coined the word. Hydroquinone is a white crystalline powder with a melting point of 173 degrees.3 Natural sources include leaves of several plants and berries in the form of arbutin. Hydrolysis of arbutin gives rise to hydroquinone. Arbutin is also found in coffee beans, teas extracted from berries, broccoli, the bark of the pear tree, red wine, wheat germ and diet cola!
In the 1940s, three chemicals - monobenzone, hydroquinone and para-hydroxypropiophenone - were chosen by investigators based on earlier publications reporting their hypopigmenting effects on skin and hair colour. Investigators observed that guinea pigs and cats exposed to hydroquinone had lightening of their fur. In vitro and in vivo tests were undertaken which showed that hydroquinone completely inhibited tyrosinase function and melanin formation, whereas propiophenone had no effect.
Initially, positive results were seen with monobenzyl ether of hydroquinone but with further tests, reports of permanent and disfiguring leucoderma became rampant. It was removed from the dermatological formulary after these reports. Currently, monobenzone is used exclusively for depigmenting patients with vitiligo too extensive to re-pigment.
Denton, Lerner, and Fitzpatrick had demonstrated that hydroquinone in vitro entirely blocked the formation of melanin when added to a solution containing tyrosinase and tyrosine, the enzyme and substrate required for melanin production. Hydroquinone had no effect on melanin formation by tyrosinase in the presence of dihydroxyphenylalanine (DOPA). The same investigators did clinical studies on hydroquinone and found it less effective than monobenzone in reducing normal or abnormal skin colour.4
In 1961, Dr. Malcolm Spencer studied hydroquinone in varying concentrations on White and African American males and concluded that skin lightened in the majority of males and more pronounced improvement was noted in white subjects compared to darker complexions. Extremely high concentrations of hydroquinone, 10-30%, were noted to deposit a dark substance on the skin produced by auto-oxidation of the molecule. These reports are the first documentations of the efficacy and safety of hydroquinone for clinical use.1
Over a period of few years, more refined or stabilized versions of hydroquinone in 2%, 3% and 5% concentrations were used in studies and histology using haematoxylin-eosin and silver nitrate stains confirmed that the quantity of melanin granules in the treated skin was reduced, without any effect on melanocytes. They also detected perivascular infiltrate in the treated skin and concluded that melanin production reduced to half of normal.1
Another group concluded that hydroquinone failed to resolve pathological hyperpigmentation completely, but results were sufficiently satisfactory to help most patients. Albert Kligman developed a new formula for lightening skin. He combined 0.1% tretinoin, 5% hydroquinone and 0.1% dexamethasone into an ointment.1 Many newer versions containing a varied concentration of the above three ingredients and newer steroids have been introduced since then and found to be effective in 90% of patients with epidermal melanin hyperpigmentation.
Mechanism of action of hydroquinone
In 1965, Arndt and Fitzpatrick proposed that hydroquinone inhibits melanin synthesis. In the period from 1984 to 1988, Penny et al. and Smith et al. suggested through studies that it might block the formation of melanin by alteration of cellular metabolism. They demonstrated that in cell cultures, hydroquinone inhibited the synthesis of DNA and RNA. The inhibition seems to be dependent on the presence and activity of tyrosinase rather than the melanin content of the cell. Thus, hydroquinone is not a useful agent for altering the colour of melanin already deposited within the epidermis or dermis. It can be used to retard or stop production of new melanin in many conditions such as melasma or post-inflammatory hyperpigmentation as further supported by studies of Grimes et al. in 1995.5
Study of the auto-oxidation: learning from history!
The earliest studies using 1.5-2% hydroquinone were limited due to auto-oxidation of hydroquinone in the ointment used. The cream used to turn from yellow to dark grey in a few weeks of use. It was felt that this colour change was the result of auto-oxidation of the available hydroquinone, making it worthless for therapy.6
The research was done to bring in more stabilized forms of hydroquinone. Once the stability was achieved the study was extended to check on the effects of various concentrations on the hyperpigmentation.
Studies showed that the effectiveness of hydroquinone reduce proportionately with the ease of auto-oxidation. The lower the concentration of hydroquinone in the ointment the lesser chance of oxidation. The higher concentration had a high chance of oxidation and subsequent change in the colour of the ointment observed. Stabilizing agents like sodium bisulphite and packaging the product in opaque and smaller tubes reduce the oxidation.
Depigmentation caused by hydroquinone is transient and not progressive. It can occur without an inflammation and even at lower concentrations of 2-3%. The sign of an initial inflammation or sensitivity of the skin is a reassurance that depigmentation will be achieved.
Depigmentation of the lighter lesions is more conspicuous than the darker ones and hence if treatments are started early for pigmentation dermatoses there is a higher chance of better cosmetic results. One especially important point to note from various studies is that repigmentation always occurs after treatment is stopped. Sunlight plays an important role in reducing the effectiveness of hydroquinone and hence it is essential to use sunscreen during therapy with hydroquinone. It is practical to use sunscreen in the morning and hydroquinone at night for the same reason.
History of USFDA rulings for hydroquinone
In 1982, the United States Food and Drug Administration (USFDA) published a tentative final monograph for over-the-counter skin-bleaching agents. It proposed that hydroquinone in concentrations of 1.5-2% be considered generally regarded as safe and effective (GRASE) as an active ingredient in over-the-counter (OTC) skin-bleaching drug products. These were in the category 1 classification of over-the-counter products.
In 2006, the USFDA proposed changing the classification of existing OTC skin-bleaching agents in category 2 as misbranded and not GRASE due to reported concerns about possible carcinogenicity and a link to ochronosis. The proposed rule at the time stated that not only would hydroquinone products that are available over-the-counter no longer be allowed to be marketed, but also all prescription hydroquinone products would have to be approved by the FDA, either through a new drug application for branded drugs or an abbreviated new drug application for generic drugs.
Products containing hydroquinone more than 1% were banned in the European Union in 2006 because studies in mice have shown the ingredients to be carcinogenic and contain trace amounts of mercury. There is some controversy as to whether the absorption level by humans has the same effect. Similar verdicts are to be proposed in many African and Asian countries.
On March 27, 2020, under the US CARES (Coronavirus Aid, Relief, and Economic Security) Act in response to the economic fallout of the COVID-19 pandemic in the United States, it has now been made it illegal to market over the counter hydroquinone products of any concentration.7
Toxicology and carcinogenicity
In the 1990’s, the only warning used by the European photography industry was allergic skin reaction because of contact with photographic developing solutions. Hydroquinone is known to destroy or inhibit melanoma cells and hence does not contribute to skin cancer. Recent articles indicate that hydroquinone causes low levels of toxicity and regulatory authorities in Germany, Scandinavian countries, the Netherlands and the European Union have classified it as a suspected carcinogen.3
Clinical safety of hydroquinone
The banning of hydroquinone in over-the-counter products in Europe was due to the concerns of ochronosis and not due to the potential carcinogenic potential. The fear of hydroquinone causing permanent discolouration and ochronosis were the main reasons behind the ban. Ochronosis is caused by the over-application of the hydroquinone. It is the duration and the percentage of the hydroquinone used that determines the occurrence of ochronosis. Ochronosis was first reported by Findlay et al. in South African Bantu women who applied hydroquinone in high concentrations and for a long period of time.10 Most of the cases reported in the USA are from the use of 2% hydroquinone.11 Hydroquinone is a major metabolic by-product of benzene. Benzene is of known leukaemogenic potential. Studies of hydroquinone in rodents and culture have shown potential damage to the cellular DNA. However, it is worthwhile to know that over the last 5 decades, no cases of internal malignancy or skin cancer have been reported in scientific literature. The other reported complications of hydroquinone application are irritant or allergic contact dermatitis, post-inflammatory hyperpigmentation, hypopigmentation and nail discolouration. Conjunctival melanosis and corneal degeneration have been reported secondary to the atmospheric exposure to hydroquinone in industries.11
Conclusion
Hydroquinone is one of the best agents we dermatologists have to counter hyperpigmentary disorders. With judicious use, hydroquinone can give particularly good results. The use should be restricted to dermatologists only. Over-the-counter use of hydroquinone may increase the risks of side effects. From early 19th century till the 21st century, as shown in Figure 1 which shows the timeline of the history of hydroquinone, this molecule was a promising agent in the fight against hyperpigmentation and is currently under the scanner of regulatory authorities.
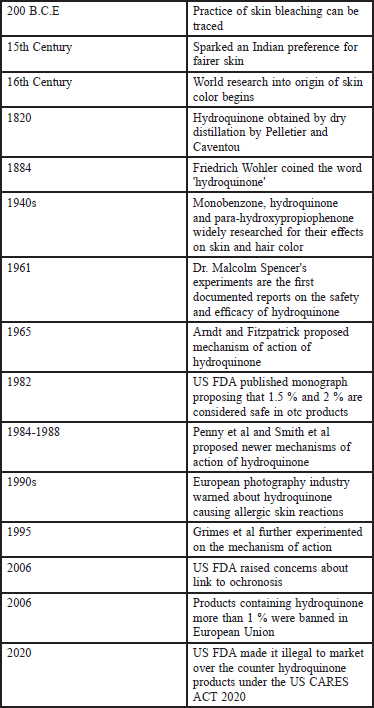
- Timeline of the history of hydroquinone.
The mutagenic and carcinogenic effects of hydroquinone remain unproven till now. The worst side-effect ever published with topical hydroquinone is ochronosis, which is rare in North America but becoming quite common in Asia and Africa, where it is marketed in high concentrations and can be used without supervision for many years.
There are several newer skin lightening products being introduced such as licorice, arbutin, kojic acid and many more as alternatives to hydroquinone.
With a review of the publications on hydroquinone safety and toxicity, we can assume that hydroquinone is safe if used in the proper concentration, with medical prescription and supervision.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Hyperpigmentation: Its historical treatment and the development of hydroquinone. Pigmentary Disorders. 2015;2:11.
- [CrossRef] [Google Scholar]
- Overview of skin bleaching history and origins. Dermatology. 2020;237:1-3.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibition of melanin formation by chemical agents. J Invest Dermatol. 1952;18:119-35.
- [CrossRef] [PubMed] [Google Scholar]
- A History of the Science of Skin Pigmentation. In: Pigmentary System. USA: Oxford, Blackwell Publishing; 2006.
- [Google Scholar]
- Topical use of hydroquinone for depigmentation. JAMA. 1965;194:962-64.
- [CrossRef] [PubMed] [Google Scholar]
- Where is the Hydroquinone? Regulatory Change Hinders Access. Practical Dermatology. Available at: Accessed October 29, 2020
- 2019 Coloring in the Gaps of Title VI: Clarifying the Protections Against the Skin-Color Caste System Georgetown University Law Center, Barnard College, Columbia University, B.A
- Skin color and colorism: Global research, concepts, and measurement. Annu Rev Sociol. 2017;43:405-24.
- [CrossRef] [Google Scholar]
- Exogenous ochronosis and pigmented colloid milium from hydroquinone bleaching creams. Br J Dermatol. 1975;93:613-22.
- [CrossRef] [PubMed] [Google Scholar]
- The safety of hydroquinone. J Eur Acad Dermatol Venereol. 2006;20:781-7.
- [CrossRef] [PubMed] [Google Scholar]





