Translate this page into:
Hormonal profile and polycystic ovaries in women with acne vulgaris
2 Department of Radiology, GMC Srinagar, Jammu and Kashmir, India
Correspondence Address:
Zubair Abdullah
Room No. 413, Surgeons Hostel Opposite Emergency Section SMHS Hospital Srinagar, Jammu and Kashmir
India
| How to cite this article: Abdullah Z, Masood Q, Hassan I, Kirmani O. Hormonal profile and polycystic ovaries in women with acne vulgaris. Indian J Dermatol Venereol Leprol 2013;79:422-424 |
Sir,
Polycystic ovarian syndrome (PCOS) has since its description by Stein and Leventhal in 1935, become one of the commonest disorders in women affecting 5-10% in the reproductive age group. [1],[2] The disorder manifests as hirsutism, obesity, menstrual disturbances, acne vulgaris, male pattern baldness, recurrent abortion, infertility, anovulation, psychosocial, and psychosexual morbidity. [3] Acne vulgaris is a self-limiting disease that affects the sebaceous follicles. It is a multifactorial disorder. Some important pathogenic factors involved include hyperkeratinization and obstruction of the sebaceous follicles as a result of abnormal keratinization of the infundibular epithelium, androgenic stimulation of sebaceous glands, and microbial colonization of pilosebaceous units by Propionibacterium acnes and subsequent perifollicular inflammation. [4]
Previous studies have shown that androgenic hormonal balance may be disturbed to some degree in about 50-75% of female-acne patients. [5] PCOS is the most frequent hormonal disease associated with acne and this can be detected by ultrasonography and measurement of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and serum testosterone levels in these patients. Most of these patients have no other clinical features of the syndrome that consists of hirsutism, infertility, or irregular menstruation. This study was performed to assess the hormonal abnormalities and polycystic ovaries (PCO) in women with acne vulgaris of ethnic Kashmiri origin.
One hundred fifty female patients of Kashmiri ethnic origin in the age group of 16-35 years with acne vulgaris were included in the study. Patients with hirsutism, menstrual abnormalities, history of intake of oral contraceptive pills, and body mass index of >25.0 were excluded from the study. Control group consisted of 150 age-matched female patients who attended out-patient department for some unrelated disorder. The study was approved by the ethical committee of the hospital. Each patient and control received a detailed clinical examination and underwent a relevant laboratory evaluation. Acne vulgaris in cases was graded using William J Cunliffe grading system into four grades. [6] Blood samples for hormonal assessment were obtained in the follicular phase. Levels of LH, FSH, total testosterone, prolactin, and androstenedione were determined. All hormonal measurements were carried out using ELISA kit (Monobind Inc., USA; and Equipar Diagnostica, Italy). Transabdominal ultrasonography was performed in follicular phase by a radiologist using Siemens Sonoline Adara with 3.5-Hz convex electronic probe. The diagnosis of polycystic ovaries was made if 10 or more follicles each 2-8 mm diameter were present in the ovarian periphery and stoma was echodense [Figure - 1]. Statistical package for the social sciences, version 10.0 was used for analysis of data. Correlation was performed using Pearson′s test. Categorical variables were compared by using Chi-square test and for continuous variables t-test was used for comparing two groups. Two-tailed significance was calculated and P0 < 0.05 was considered significant.
In 60% of cases, age of onset of acne was between 16 to 19 years. Grade 1 acne was present in 17.5%, grade 2 in 46.0%, grade 3 in 33.5%, and grade 4 in 3%. Hormonal abnormalities were found in 36% of patients as compared to 9% in controls. Polycystic ovaries on ultrasonography were found in 28.8% of cases as compared to 9.3% in controls. A statistically significant difference (P=0.000) was found between the two groups. Sixty-seven percent of patients with hormonal abnormalities had polycystic ovaries on ultrasonography. No statistically significant difference in the mean serum levels of FSH (p =0.264) and prolactin (p =0.679) was found between the two groups. The mean serum levels of LH (p = 0.001), testosterone (0.000), and androstenedione ( p = 0.000) were significantly greater in cases as compared with the controls [Table - 1]. No statistically significant difference was found in the hormonal abnormalities and ultrasonographic evidence of polycystic ovaries between various grades of acne.
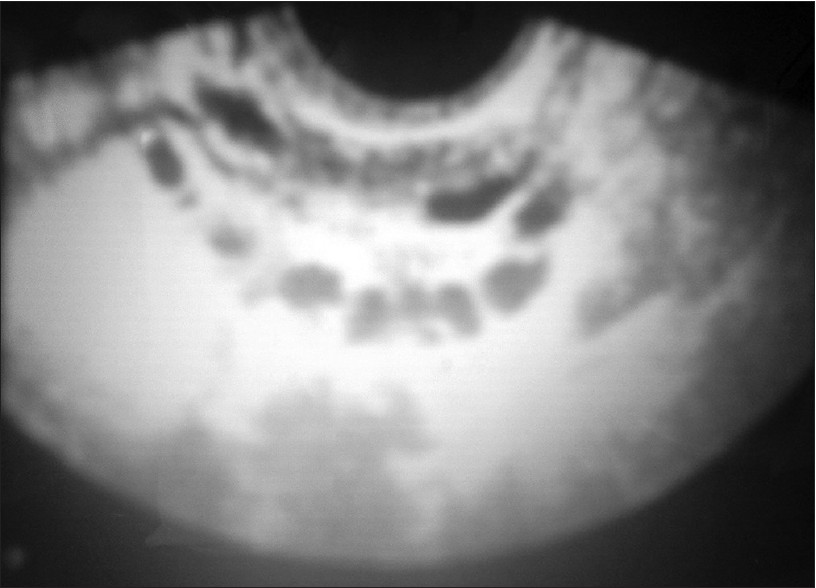 |
| Figure 1: Ultrasonography (USG) showing polycystic ovaries |
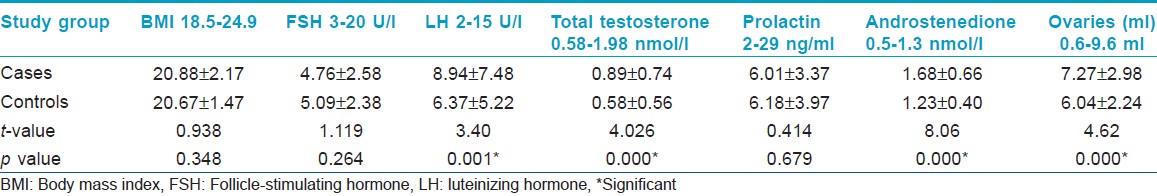
In this study, 36% of acne females had at least one abnormal (greater than the upper limit) biochemical marker of PCOS, that is raised serum LH, raised LH/FSH ratio, or raised testosterone. Serum FSH and serum prolactin levels were normal in both patients and controls, with LH, testosterone, and androstenedione having significantly higher mean levels in acne patients than controls. [7] Vexiau, et al. [8] has reported hyperandrogenism in 86% of patients with persistent acne without signs of virilism. Slayden, et al. [9] reported abnormal levels of androgenic markers in 76% and 55% of patients with adult onset acne, respectively. As our population was not confined to persistent acne or adult onset acne, hormonal abnormalities were found in 36% of patients.
In conclusion, this study demonstrates a higher evidence of polycystic ovaries and androgenic hormone abnormalities in females with acne compared with females without acne. Moreover, polycystic ovaries are common in women with acne and not necessarily associated with menstrual disorders, obesity, or hirsutism and that these patients can be considered as a part of the clinical spectrum of fully developed PCOS.
| 1. |
Stein LF, Leventhal MC. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol 1935;29:181-91.
[Google Scholar]
|
| 2. |
Franks S. Polycystic ovary syndrome. N Eng J Med 1995;333:853-61.
[Google Scholar]
|
| 3. |
Tsilchorozidou T, Overton C, Conway GS. The pathophysiology of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004;60:1-17.
[Google Scholar]
|
| 4. |
Toyoda M, Morohashi M. New aspects in acne inflammation. Dermatology 2003;206:17-23.
[Google Scholar]
|
| 5. |
Sheehan-Dare RA, Hughes BR, Cunliffe WJ. Clinical markers of androgenicity in acne vulgaris. Br J Dermatol 1988;119:723-30.
[Google Scholar]
|
| 6. |
Cunliffe WJ, Gollnick HP. Acne: Diagnosis and management. J R Soc Med 2001;94:652.
[Google Scholar]
|
| 7. |
Förström L, Mustakallio KK, Dessypris A, Uggeldahl PE, Adlercreutz H. Plasma testosterone levels and acne. Acta Derm Venereol 1974;54:369-71.
[Google Scholar]
|
| 8. |
Vexiau P, Husson C, Chivot M, Brerault JL, Fiet J, Julien R, et al. Androgen excess in women with acne alone compared with women with acne and/or hirsutism. J Invest Dermatol 1990;94:279-83.
[Google Scholar]
|
| 9. |
Slayden SM, Moran C, Sams WM Jr, Boots LR, Azziz R. Hyperandrogenemia in patients presenting with acne. Fertil Steril 2001;75:889-92.
[Google Scholar]
|
Fulltext Views
4,733
PDF downloads
1,934





