Translate this page into:
Human herpesvirus-8 and human cytomegalovirus infections in Bowen's disease: Is there any association?
2 Department of Dermatopathology, Razi Hospital, Tehran University of Medical Sciences, Tehran, Iran
Corresponding Author:
Hamideh Moravvej
Skin Research Center, Shahid Beheshti University of Medical Sciences, Shohada-e-Tajrish Hospital, Shahrdari Street, 1989934148 Tehran
Iran
hamidehmoravej@sbmu.ac.ir
| How to cite this article: Abdollahimajd F, Moravvej H, Rashidi A, Aref S, Safaei-Naraghi Z. Human herpesvirus-8 and human cytomegalovirus infections in Bowen's disease: Is there any association?. Indian J Dermatol Venereol Leprol 2017;83:372-373 |
Sir,
Bowen's disease is an in situ squamous cell carcinoma confined to the epidermis, which has several etiological factors viz., irradiation, carcinogens, immunosuppression and viral infections such as human papillomavirus.[1] The role of human herpesvirus-8 and human cytomegalovirus in its etiopathogenesis is still unclear. Human herpesvirus-8, a gamma herpesvirus, is implicated in almost all cases of Kaposi's sarcoma.[2] Other related neoplasms include multicentric Castleman's disease and primary effusion lymphoma. Lytic replication plays a pivotal role in human herpesvirus-8 tumorigenesis, but the precise mechanism of this association is unclear.[3] Some studies have proposed that human herpesvirus-8 infection increases the risk of proliferative cutaneous disorders like actinic keratosis, squamous cell carcinoma, basal cell carcinoma and seborrheic keratosis,[4] but the results are contradictory. Nishimoto et al (1997) detected human herpesvirus-8 sequences in 61.9% of Bowen's disease samples from immunocompetent patients.[4] In contrast, Mitsuishi et al., depicted that human herpesvirus-8 DNA is not involved in the pathogenesis of Bowen's disease in such patients.[5]
Human cytomegalovirus, a beta herpesvirus, is an important cause of morbidity and mortality in lung transplant recipients. Pathologic data demonstrate that human cytomegalovirus infections may be involved in the etiology of several human malignancies such as carcinoma of the oral cavity. The oncogenic potential of the virus have been well demonstrated in vitro. This virus inhibits apoptosis and facilitates malignant transformation. However, till now no definitive evidence supports this association. Zafiropoulos et al. detected human cytomegalovirus DNA in 42 (38.5%) of 109 skin lesion specimens (non-melanoma skin cancers including squamous cell carcinoma, basal cell carcinoma and Bowen's disease);[6] however in the study by Zhang et al. no human cytomegalovirus DNA was found in either esophageal cancer or normal mucosa samples.[7]
Consequently, we designed this study to evaluate the potential association of human herpesvirus-8 and human cytomegalovirus infections with Bowen's disease in skin samples. All the samples were identified as Bowen's disease after testing in double-blinded conditions. Evaluation was performed using real-time polymerase chain reaction assay on a total of 53 paraffin-embedded lesional skin specimens including 36 non-immunosuppressed patients with Bowen's disease (M:F 3:1) [25 from sun-exposed and 11 from non-sun-exposed sites]; 7 Kaposi's sarcoma patients (M:F 1.33:1) as positive controls and 10 subjects with chronic dermatitis (M:F 1:1) as negative controls for human herpesvirus-8 infection were also tested. 10 known human cytomegalovirus-positive specimens (M:F 3:7) and 14 known human cytomegalovirus-negative specimens (M:F 5:9) served as positive and negative controls for human cytomegalovirus, respectively. The tissue samples were thawed at room temperature, spun and processed according to kit instructions (CMV Kit: Real-TM, Sacace Biotechnology, Catalog No. V7-100sRT; HHV-8 Kit: Real-time PCR Kit Liferiver Tm-Catalog No. OD-0212-02). To prevent possible cross-contamination between samples during polymerase chain reaction, we used disposable microtome blades to cut each paraffin block. We extracted the entire DNA using a High-pure PCR Template Preparation Kit (Roche method) from samples according to the manufacturer's instructions. TaqMan-based real-time polymerase chain reaction (sensitivity: 100 copy/ml) was conducted using an Applied Biosystems StepOne instrument. Reactions were conducted in a final volume of 25 μl. The results were positive or negative based on laboratory-determined cut off value. Statistical analysis was performed with SPSS 2.0 (Statistical Package for Social Science Inc., Chicago, IL, USA). Where appropriate, the two-sided Fisher exact test for tabulated categorical data was used to analyze the differences between the negative control group (chronic dermatitis specimens and the human cytomegalovirus-negative control group) and the other diseases investigated with regard to the frequencies of human herpesvirus-8 and cytomegalovirus positivity. P value of 0.05 or lower was statistically significant. According to the results of real-time polymerase chain reaction on 53 paraffin-embedded blocks, 5 (13.9%) and 3 (8.3%) cases of the Bowen's disease samples showed positive results for the human herpesvirus-8 and human cytomegalovirus genomes, respectively. These results were not statistically significant [Table - 1] and [Table - 2].

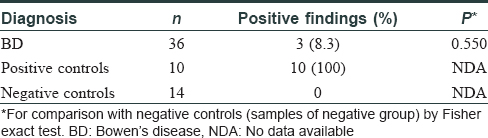
We could not perform immunohistochemical staining, serological tests to detect anti-human herpesvirus-8 and anti-human cytomegalovirus antibodies or serum viral DNA load assessment due to limited facilities at our centre. Although our results were not statistically significant, human herpesvirus-8 and human cytomegalovirus genome was detected in some samples of Bowen's disease. Thus, we shall need further studies with a larger sample size with measurement of the serum viral DNA load or detection of antibodies against human herpesvirus-8 and human cytomegalovirus to establish the causal relationship between these viral infections and Bowen's disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
| 1. | Neubert T, Lehmann P. Bowen's disease – A review of newer treatment options. Ther Clin Risk Manag 2008;4:1085-95. [Google Scholar] |
| 2. | Moravvej H, Aref S, Keyvani H, Abolhasani E, Sarrafi Rad S, Jafari R. Association of mycosis fungoides and large plaque parapsoriasis with human herpes virus 8. J Skin Stem Cell 2014;1:e21562. [Google Scholar] |
| 3. | Gantt S, Casper C. Human herpesvirus 8-associated neoplasms: The roles of viral replication and antiviral treatment. Curr Opin Infect Dis 2011;24:295-301. [Google Scholar] |
| 4. | Nishimoto S, Inagi R, Yamanishi K, Hosokawa K, Kakibuchi M, Yoshikawa K. Prevalence of human herpesvirus-8 in skin lesions. Br J Dermatol 1997;137:179-84. [Google Scholar] |
| 5. | Mitsuishi T, Sata T, Matsukura T, Kawashima M. Human herpesvirus 8 DNA is rarely found in Bowen's disease of non-immunosuppressed patients. Br J Dermatol 1997;136:803-4. [Google Scholar] |
| 6. | Zafiropoulos A, Tsentelierou E, Billiri K, Spandidos DA. Human herpes viruses in non-melanoma skin cancers. Cancer Lett 2003;198:77-81. [Google Scholar] |
| 7. | Zhang DH, Zhang QY, Hong CQ, Chen JY, Shen ZY, Zhu Y. Prevalence and association of human papillomavirus 16, Epstein-Barr virus, herpes simplex virus-1 and cytomegalovirus infection with human esophageal carcinoma: A case-control study. Oncol Rep 2011;25:1731-8. [Google Scholar] |
Fulltext Views
2,002
PDF downloads
1,723





