Translate this page into:
Hydroa vacciniforme-like lymphoproliferative disorder in Ecuadorian children: A case series
Corresponding author: Dr. John Jairo Dávila-Rodríguez, Department of Dermatologic Surgery, Dermatologic Institute of Jalisco Dr. José Barba Rubio, Guadalajara, Mexico. dr.davila.dermato@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Garzón E, Dávila-Rodríguez JJ. Hydroa vacciniforme-like lymphoproliferative disorder in Ecuadorian children: A case series. Indian J Dermatol Venereol Leprol 2023;89:403-7.
Abstract
We report the clinical and histopathological features of hydroa vacciniforme-like lymphoproliferative disorder in five indigenous and Mestizo children. All the children resided at higher altitudes, experiencing maximal solar exposure. All cases presented with prurigo along with Epstein-Barr virus infection. Histopathologic examination showed an atypical, CD30 + lymphocytic infiltrate with angiocentricity in all, while three cases demonstrated panniculitis-like infiltrate.
Keywords
Cutaneous lymphoma
Epstein–Barr virus
hydroa vacciniforme
Introduction
Hydroa vacciniforme-like lymphoproliferative disorder represents 5.4% of primary cutaneous T-cell lymphomas according to a Peruvian study.1 It could represent the malignant end of a spectrum of Epstein–Barr virus-associated hydroa vacciniforme, possibly affecting genetically predisposed individuals.2 Unlike classical hydroa vacciniforme, these lyphoproliferative skin lesions are distributed on both sun-exposed and sun-protected skin areas and tend to worsen with age, developingextensive necrosis and disfigurement.3 Clinically, classic examples of hydroa vacciniforme-like lymphoproliferative disorder usually present with vesicles, blisters, erythema, ulcerations, crusts, varioliform scars and edema predominantly involving the face and upper extremities.4 Hydroa vacciniforme-like lymphoproliferative disorder usually follows an aggressive and fatal clinical course.5
Case Report
The clinical features of each case are summarized in Table 1. Of the five children, two were Mestizos, while the remaining three were of indigenous ethnicity. Notably, all patients resided at high altitude provinces of the Andean region which experienced extremely high level of ultraviolet radiation index. Furthermore, a low socioeconomic agrarian background resulted in prolonged, unprotected sun-exposure. Interestingly, all patients reported an exaggerated reaction to insect bites with clinical lesions resembling prurigo. All patients presented with skin lesions in both sun-exposed and non-sun-exposed areas including painful papulovesicular eruption, ulceration, crusts and varioliform scars located on the face, trunk and extremities [Figure 1].
| Patient | Age (years) | Sex | Ethnic identity | Preexisting medical conditions | Distribution of lesions | Symptoms duration, (year) | Features of lesions | Systemic involvement* | Treatment + | Follow-up | Current status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | Female | Indigenous | Prurigo | Face, trunk and extremities | 6 | Periorbital edema, papulovesicles and crusts. Large ulcers on abdomen. Erythematous annular plaques on legs | Yes | CHOP, 3 cycles | 6 months, rapid deteriorationand multiorgan failure due to hemophagocytic syndrome | Dead |
| 2 | 12 | Female | Mestizo | Prurigo | Face and extremities | 4 | Papulovesicles, crusts and scars | Yes | CHOP, 6 cycles | 2 years, remission and recurrences | Alive |
| 3 | 10 | Male | Indigenous | Prurigo | Face and extremities | 2 | Papulovesicles, crusts, scars and lips erosions | No | CHOP, 4 cycles | 8 months, rapid deterioration | Dead |
| 4 | 15 | Female | Indigenous | Prurigo | Face, trunk and extremities | 3 | Marked facial edema with exuberant superior lip involvement. Scars, ulcers and crusts. Erythematous corymbiform plaques on legs | Yes | CHOP, 1 cycle | 2 months, rapid deterioration, development of hemophagocytic syndrome | Dead |
| 5 | 5 | Male | Mestizo | Prurigo | Face and extremities | 3 | Scars and crusts | No | CHOP, 6 cycles | 4 years remission and recurrences | Alive |
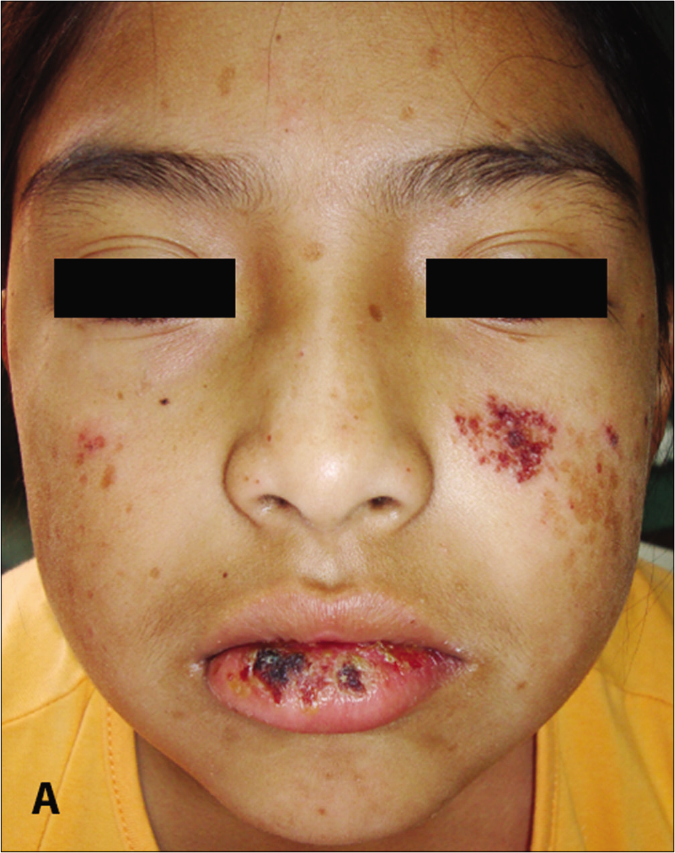
- Patient 3: Papulovesicles, scars and lips erosions
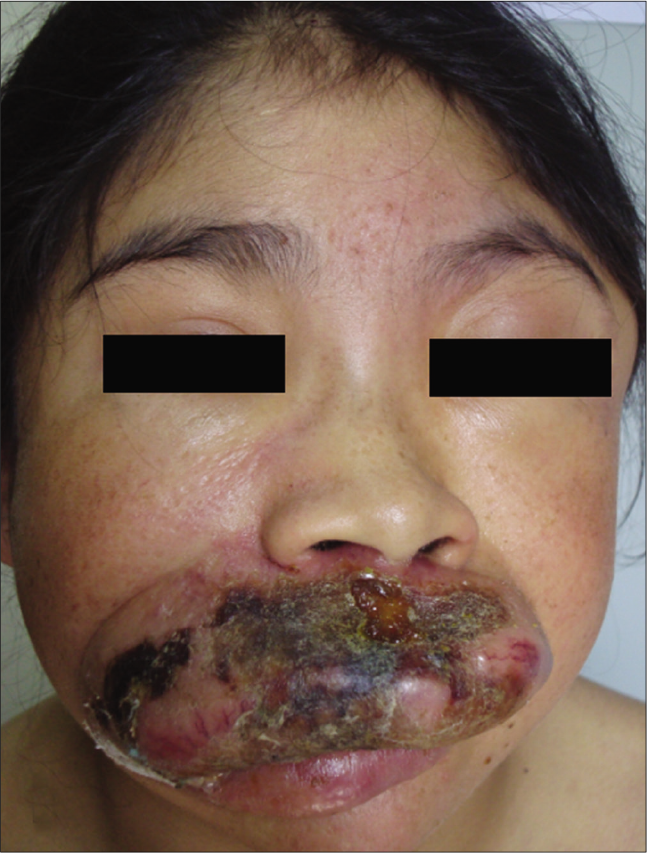
- Patient 4: Exuberant superior lip involvement
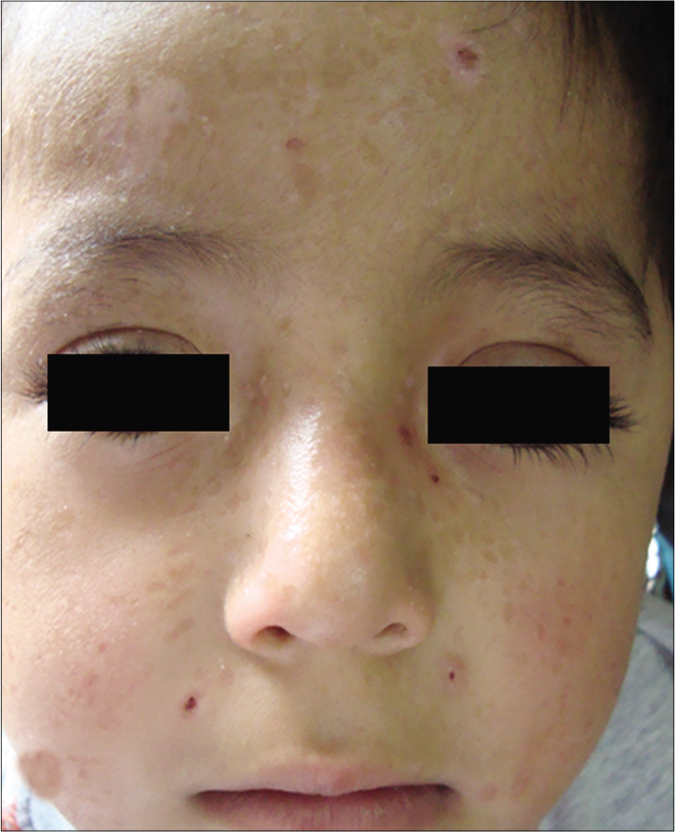
- Patient 5: Varioliform scars
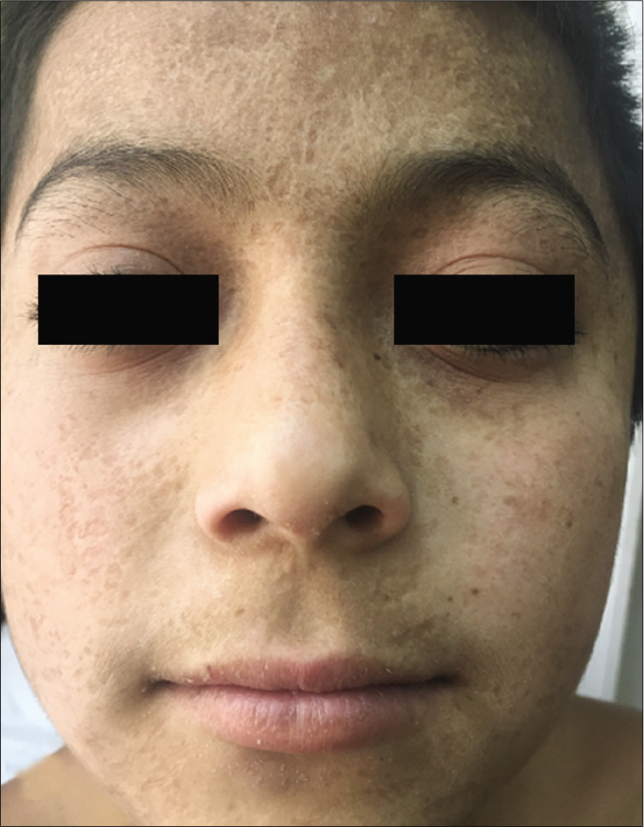
- Patient 5: Four years later
The laboratory and histopathological features of patients are summarized in Table 2. All cases were associated with Epstein-Barr virus infection. Histopathologically, the infiltrate demonstrated small- to medium-sized atypical lymphocytes. Each call showed enlarged, round to oval and pleomorphic nuclei with fine chromatin and inconspicuous nucleoli along with moderate pale stained cytoplasm. All cases exhibited angiotropism/angiocentricity, and a predominant diffuse and periadnexal pattern without epidermotropism. Three cases demonstrated subcutaneous infiltration. Immunohistochemically, the neoplastic lymphocytes were CD20- and all cases showed a 30–40% staining with CD30, four cases showed a cytotoxic profile CD8+, and one was CD4+ [Figure 2]. There was no expression of natural killer-cell-associated antigens (CD56-). T-cell receptor-gamma, Epstein-Barr virus encoded small RNAs (EBER) and clonality studies were not performed. After confirmation of diagnosis, they were referred to oncology for treatment with skin lesions being monitored every 3 to 6 months by a dermatologist. Two patients survived with persistent disease.
| Patient | Laboratory | Histopathological and immunohistochemical | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CBC | ESR | LFT | EBV serology | Infiltrate pattern | Angiotropism | Panniculitis-like | CD4 | CD8 | CD30 | CD56 | |
| 1 | Anemia, leukopenia and thrombocytopenia | ↑ | Abnormal | + | Perivascular and periadnexal | + | + | - | + | + | - |
| 2 | Anemia and leukopenia | ↑ | Abnormal | + | Diffuse and periadnexal | + | - | - | + | + | - |
| 3 | Normal | ↑ | Normal | + | Diffuse | + | - | + | - | + | - |
| 4 | Anemia, leukopenia and thrombocytopenia | ↑ | Abnormal | + | Diffuse and periadnexal | + | + | - | + | + | - |
| 5 | Normal | ↑ | Normal | + | Diffuse | + | + | - | + | + | - |
CBC=complete blood count, ESR=erythrocyte sedimentation rate, LFT=liver function test, EBV=Epstein–Barr virus
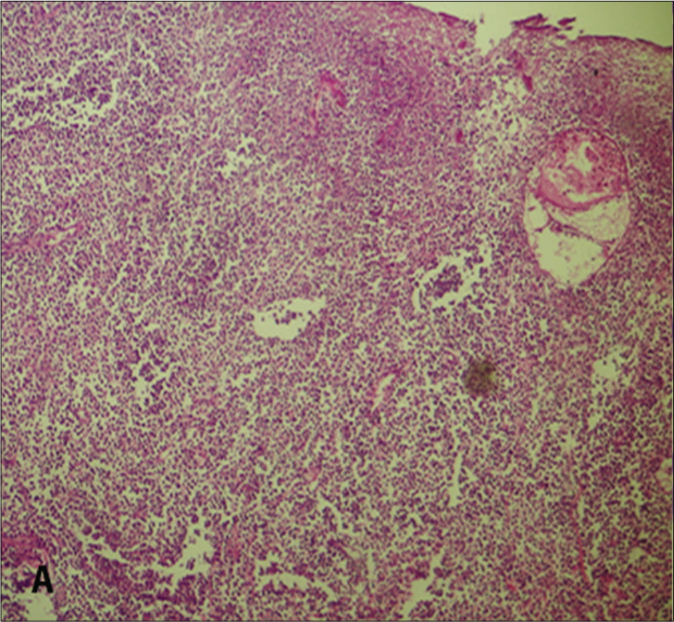
- Diffuse dermal infiltration of lymphocytes (H&E, ×100)
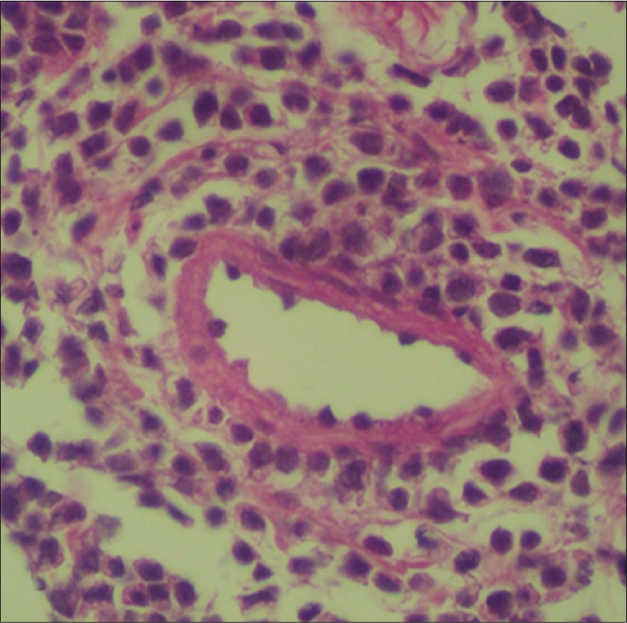
- Atypical lymphocytes with angiotropism (H&E, ×100)
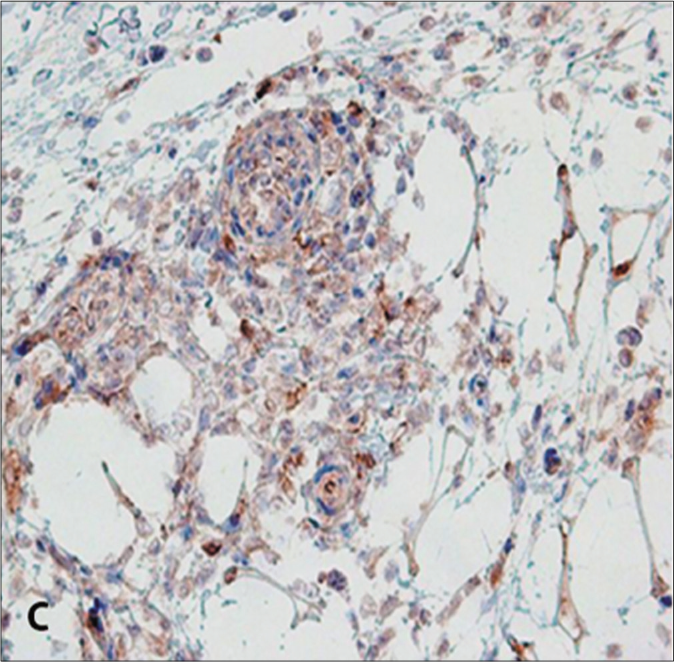
- CD 30 expression in neoplastic cells (CD30 immunohistochemistry, ×100)
Discussion
Initially, hydroa vacciniforme-like lymphoproliferative disorder was considered as a variant of extranodal natural killer/T-cell lymphoma, nasal type.2,6 Later, it was grouped among the Epstein–Barr virus lymphoproliferative disorders of childhood, as it predominantly involves children and young adults of Latin American and Asian descent. 3,7 There is a strong association with latent Epstein–Barr virus infection, as detected by a polymerase chain reaction in 80% to 100% of hydroa vacciniforme-like lymphoproliferative disorder patients.1 Serological memory for Epstein–Barr virus is valuable in developing countries like Ecuador, where EBER and T-cell receptor-gene clonality studies are limited due to economic and technological constraints. Epstein–Barr virus-associated T/natural killer-cell lymphoproliferative disorders are rare and usually occur in apparently immunocompetent patients, closely linked to chronic infection.4 So-called chronic active Epstein–Barr virus infection comprises heterogeneous disorders, including hydroa vacciniforme, hypersensitivity to mosquito bites, Epstein–Barr virus-associated hemophagocytic syndrome, natural killer/T-cell lymphoma or leukemia and hydroa vacciniforme-like lymphoproliferative disorder.8
A constant feature of hydroa vacciniforme-like lymphoproliferative disorder is the ethnicity of patients, who are of indigenous or Mestizo ancestry. This ethnicity is shared by Mexican, Peruvian, Bolivian and Asian patients probably due to the east Asian origin of the population in large areas of Latin America; thus, domicile may be a contributory factor for developing hydroa vacciniforme-like lymphoproliferative disorder.5-7 The restriction of this disease to some ethnic groups strongly suggests a genetic background, probably related to the immune response to Epstein–Barr virus.7
Hydroa vacciniforme-like lymphoproliferative disorder has some common clinical features including facial edema, recurrent papulovesicular eruption progressing to necrosis and varioliform scars, blisters, ulcers, crusts and subsequent systemic involvement such as intermittent fever, hepatosplenomegaly and lymphadenopathy.9 Clinical worsening is heralded by a lack of improvement, severe facial and lip swelling, systemic complications and episodes of hypersensitivity to mosquito bites, characterized by necrotic skin lesions and generalized symptoms subsequent to mosquito bites.5 We propose preexistent prurigo to be an important clinical marker for this disease. Furthermore, we noted systemic involvement to be disproportional to dermatological manifestations, so patients with incipient lesions succumbed despite oncological treatment. These patients possibly suffered from complications of both chemotherapy and worsening T-cell neoplastic disease, resulting in multi-organ failure due to hemophagocytic syndrome. Our differentials included other inflammatory, autoimmune and neoplastic conditions such as chronic active infections, lupus erythematosus and other hematological malignancies.9 Interestingly, prominent persistent facial edema can also be observed in extranodal natural killer/T-cell lymphoma, nasal type.7
Histologic evaluation revealed atypical lymphoid infiltrate in the dermis and subcutis, showing perivascular and periadnexal distribution and angiocentric/angioinvasive pattern.6,9 Although epidermal necrosis is frequent, we observed no or minimal epidermotropism in most cases.7 Phenotypically, most patients revealed a T-cell phenotype with divergent immunoexpression of either CD4 or CD8; however, majority of Latin American patients have a cytotoxic profile (CD8+).4 Neoplastic T cells are CD3+, CD45+, CD8+/−, CD4-/+, T-cell receptor αβ or γδ+ T cells; a small proportion of cases have been reported to have a natural killer-cell phenotype (CD56), suggestive of a T-cell or a natural killer-cell lineage.2,4,6 The lymphoid cells, regardless of cell-type derivation, are positive for cytotoxic markers such as granzyme B and TIA-1.8 CD30 expression was more common in a natural killer-cell phenotype and tended to involve the subcutaneous tissue with rimming of the neoplastic cells surrounding individual fat cells, mimicking subcutaneous panniculitis T-cell lymphoma.2 Interestingly, some studies have proposed hydroa vacciniforme-like lymphoproliferative disorder -to be be more commonly associated with a panniculitic involvement and a natural killer-cell phenotype; however, none of our patients had a natural killer-cell phenotype, although three showed a panniculitis-like infiltrate.
Currently, there is no standard therapy for patients with hydroa vacciniforme-like lymphoproliferative disorder. Beltrán et al.10 used thalidomide in four patients and reported 80% lesional improvement with milder side effects, compared to chemotherapy and radiation therapy. Other therapies, such as prednisolone, bone marrow or allogeneic hematopoietic stem cell transplantation, interleukin-2, cyclosporine A, antibody to CD20, antiviral agents and chloroquine have resulted in temporary remission or symptomatic improvement2,6,8
All the current patients were of indigenous and Mestizo ethnicity and came from a high altitude region with maximal solar exposure, suggesting a combination of genetic and environmental factors. Notably, facial edema is not always present. Furthermore, all our patients had prurigo which highlights the association of this lymphoma with Epstein– Barr virus-associated cutaneous hypersensitivity to mosquito bites. Interestingly, three patients showed a panniculitis-like infiltrate with CD56- and CD30+, contrasting the widespread notion that that subcutaneous involvement tends to have a natural killer-cell (CD56+) phenotype.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Primary cutaneous T-cell lymphoma: Experience from the Peruvian National Cancer Institute. An Bras Dermatol. 2017;92:649-54.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroa vacciniforme-like T-cell lymphoma: A further Brazilian case. Am J Dermatopathol. 2018;40:201-4.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathological analysis of the hydroa vacciniforme-like lymphoproliferative disorder with natural killer cell phenotype compared with cutaneous natural killer T-cell lymphoma. Exp Ther Med. 2018;16:4772-8.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroa vacciniforme-like lymphoma with primarily periorbital swelling: 7 cases of an atypical clinical manifestation of this rare cutaneous T-cell lymphoma. Am J Dermatopathol. 2015;37:20-5.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroa vacciniforme-like cutaneous T-cell lymphoma: Clinicopathologic and immunohistochemical study of 12 cases. J Am Acad Dermatol. 2013;69:112-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroa vacciniforme-like lymphoma in Tibetan children: 2 cases and a literature review. Am J Dermatopathol. 2018;40:358-61.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathologic features of hydroa vacciniforme Like lymphoma. Am J Dermatopathol. 2015;38:20-5.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroa vacciniforme-like lymphoma: A chronic EBV+lymphoproliferative disorder with risk to develop a systemic lymphoma. Blood. 2013;122:3101-10.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroa vacciniforme-like cutaneous T-cell lymphoma in a child: A case report. Medicine (Baltimore). 2018;97:13-6.
- [CrossRef] [PubMed] [Google Scholar]
- Thalidomide for the treatment of hydroa vacciniforme-like lymphoma: Report of four pediatric cases from Peru. Am J Hematol. 2014;89:1160-1.
- [CrossRef] [PubMed] [Google Scholar]






