Translate this page into:
Imatinib-induced pyoderma gangrenosum in a patient with chronic myeloid leukemia
Corresponding author: Prof. Angelo Marzano, Via Pace 9, Milan-20122, Italy. angelo.marzano@unimi.it
-
Received: ,
Accepted: ,
How to cite this article: Faraci AG, Genovese G, Ferrucci S, Marzano AV. Imatinib-induced pyoderma gangrenosum in a patient with chronic myeloid leukemia. Indian J Dermatol Venereol Leprol 2021;87:704-6.
Sir,
Pyoderma gangrenosum is an uncommon neutrophilic dermatosis presenting with sterile pustules that rapidly progress to painful skin ulcers with undermined, violaceous borders.1 Imatinib is a tyrosine kinase inhibitor used to treat certain types of cancer, including chronic myeloid leukemia and gastrointestinal stromal tumor. Herein, we present a case of severe pyoderma gangrenosum induced by imatinib.
A 59-year-old obese woman was diagnosed with chronic myeloid leukemia and started on imatinib mesylate 400 mg/ day. Over the following six months, she gradually developed painful, large and variously interconnected skin ulcers with ragged erythematous-violaceous edges, and abundant reddish discharge at their base on pubis, inguinal, perianal, and gluteal areas. Clinical examination revealed full-thickness tissue loss with exposed fascia and muscle [Figures 1a and b]. Skin histopathology demonstrated epidermal ulceration associated with a dermal-hypodermal neutrophil-rich inflammatory infiltrate [Figures 2a and b]. Wound cultures for aerobic and anaerobic bacteria or fungi yielded negative results. Laboratory studies revealed anemia, neutrophilic leukocytosis, and elevated acute-phase reactants. Polymerase chain reaction on ulcer swabs failed to detect DNA of herpes simplex viruses 1 and 2. Magnetic resonance imaging and colonoscopy ruled out underlying bone or visceral involvement and inflammatory bowel disease, respectively. A diagnosis of pyoderma gangrenosum probably induced by imatinib (Naranjo score = 5) was made and this drug was withdrawn. Immunosuppressive treatments were avoided due to the risk of chronic myeloid leukemia progression and no specific therapies were administered, apart from opioid analgesics and systemic antibiotic in addition to potassium permanganate bathing to prevent ulcer superinfection. Wounds progressively healed with residual cribriform and hypertrophic scarring, and concomitant pain relief. At six-month follow-up visit, almost complete pyoderma gangrenosum remission was observed [Figures 3a and b] and drug discontinuation was supported by chronic myeloid leukemia stability on molecular biology (BCR-ABL1 levels of 0.471%).

- Large and deep skin ulcers located on the pubis and inguinal folds of a 59-year-old woman

- Large and deep skin ulcers located on the perianal-gluteal area of a 59-year-old woman
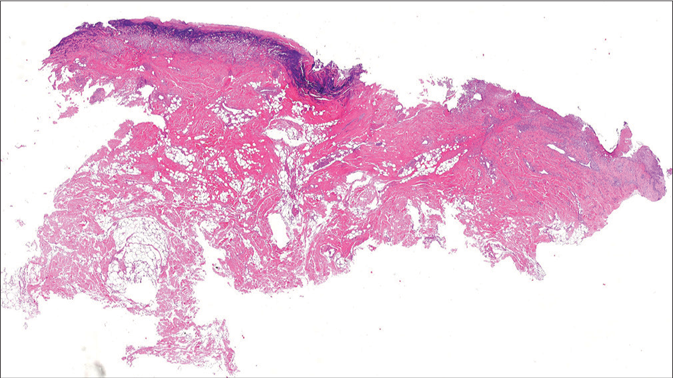
- Skin histopathology showing epidermal ulceration and a dermalhypodermal inflammatory infiltrate predominantly consisting of neutrophils (hematoxylin-eosin staining, original magnification ×10)

- Close-up view of skin histopathology showing a dermal neutrophil-rich infiltrate (hematoxylin-eosin staining, original magnification ×200)

- Almost complete remission of lesions located on the perianal-gluteal area on imatinib withdrawal at 6-month follow-up visit

- Almost complete remission of lesions located on the pubis and inguinal folds on imatinib withdrawal at 6-month follow-up visit
Pyoderma gangrenosum is a neutrophil-mediated disease rarely triggered by drugs.2 Although tyrosine kinase inhibitors, particularly sunitinib,3 may induce pyoderma gangrenosum by fostering chemokine release, vascular permeability, and neutrophil migration from peripheral blood into the skin and rarely internal organs,2 a single case of imatinib-induced pyoderma gangrenosum has been reported in a patient with gastrointestinal stromal tumour.4 Indeed, imatinib has been demonstrated either to promote myelopoiesis or to accelerate neutrophil maturation through a c-kit-dependent mechanism, with no effects on lymphopoiesis.5 Thus, the alteration of neutrophil homeostasis by imatinib might explain its pyoderma gangrenosum inducing effect. Other cutaneous reactions to imatinib include erythematous maculopapular eruptions, periorbital edema, toxic epidermal necrolysis, Stevens-Johnson syndrome, acute generalized exanthematous pustulosis, purpuric vasculitis, and mycosis fungoides-like reactions.6 The close temporal association between drug initiation and pyoderma gangrenosum onset, the severity of our patient’s lesions, and the dramatic remission following drug suspension, makes our case worth reporting. We aim to make clinicians aware of this extremely rare adverse skin reaction in patients receiving tyrosine kinase inhibitors.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Diagnostic criteria of ulcerative pyoderma gangrenosum: A Delphi consensus of international experts. JAMA Dermatol. 2018;154:461-6.
- [CrossRef] [Google Scholar]
- Drug-induced pyoderma gangrenosum: A model to understand the pathogenesis of pyoderma gangrenosum. Br J Dermatol. 2017;177:72-83.
- [CrossRef] [Google Scholar]
- Drug-induced pyoderma gangrenosum: A review. Am J Clin Dermatol. 2018;19:67-77.
- [CrossRef] [Google Scholar]
- Low doses of imatinib induce myelopoiesis and enhance host antimicrobial immunity. PLoS Pathog. 2015;11:e1004770.
- [CrossRef] [Google Scholar]
- Imatinib mesylate and dermatology part 2: A review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-31.
- [Google Scholar]





