Translate this page into:
Imatinib induced Stevens-Johnson syndrome: Lack of recurrence following re-challenge with a lower dose
2 CSI Medical College, Karakkonam, Trivandrum, India
Correspondence Address:
Keechilat Pavithran
Department of Medical Oncology, Amrita Institute of Medical Sciences, Elamakkara, Kochi-682 026
India
| How to cite this article: Pavithran K, Thomas M. Imatinib induced Stevens-Johnson syndrome: Lack of recurrence following re-challenge with a lower dose. Indian J Dermatol Venereol Leprol 2005;71:288-289 |

Sir,
Imatinib mesylate (STI-571) is a selective and potent small-molecule inhibitor of tyrosine kinases, including BCR-ABL fusion protein, c-Kit and platelet-derived growth factor receptor. It is the most active agent for the treatment of chronic myeloid leukemia (CML), and gastrointestinal stromal tumors. Cutaneous reactions to imatinib therapy are increasingly being recognized, with 5% of these reactions being severe. Though a variety of dermatological manifestations have been described, occurrence of Stevens-Johnson syndrome is rare.
A 35-year-old man was diagnosed as having Philadelphia-positive chronic myeloid leukemia in the chronic phase. He was started on imatinib 400 mg daily, which was the only medication given. His initial hemogram revealed: hemoglobin, 12.8 g/dl; white blood cell count, 248 x 109/l; and platelet count, 440x 109/l. Complete hematologic remission was achieved with imatinib in two weeks. On the 14th day of treatment, the patient developed an itchy macular eruption mainly over the trunk. Atypical target lesions were observed without areas of necrosis. In addition to the skin lesions, the mucosae were involved with ulcerative lesions. A clinical diagnosis of Stevens-Johnson syndrome was made.
Imatinib was stopped immediately and the patient was given fexofenadine and prednisolone. Healing started within a few days and in one week the lesions cleared. One week following the complete clearance of the rash imatinib was restarted at a dosage of 100 mg daily. This re-challenge at a lower initial dose did not produce any adverse cutaneous reaction. The dose of imatinib was gradually escalated to 400 mg which was continued. Presently, he is in hematologic remission without any untoward side effect.
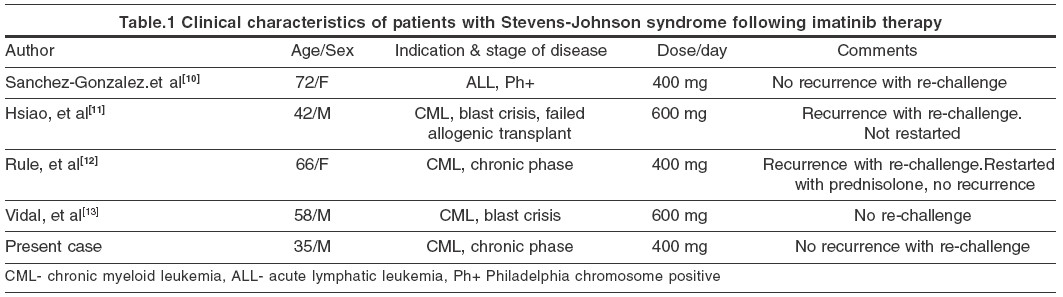
A variety of adverse cutaneous reactions have been described with imatinib. Of these, rash and edema occur most commonly, the incidence being 66.7% and 65% respectively.[1] Severe and life threatening reactions occur in 5% cases. Reports of cutaneous adverse reactions other than maculo-papular eruptions are rare with imatinib. However, it may cause acute generalized exanthematous pustulosis,[2] oral lichenoid eruption,[3] vasculitis,[4] pseudolymphoma,[5] epidermal necrolysis,[6] hypopigmentation,[7] erythema nodosum,[8] exfoliative dermatitis,[9] and Stevens-Johnson syndrome.[10],[11],[12],[13] Of the four cases of Stevens-Johnson syndrome due to imatinib reported previously, two were males and two females. All these patients, except one, had CML.[10] In two of these cases the lesions reappeared on re-challenge with imatinib.[11],[12] However, our patient, as well as one of the reported cases[10] did not have any recurrence following re-challenge. Clinical characteristics of these patients are shown in [Table - 1]. The etiology of Stevens-Johnson syndrome is not clear and drug-induced cases have been thought to be mediated by an immunological mechanism. Brouard and Saurat have shown that, the incidence of cutaneous reactions with imatinib increases with escalating doses of the drug.[14] Valeyrie et al have reported female sex and imatinib dosage as being independent risk factors for the development of rashes in a multivariate analysis.[1]
As the indications and use of imatinib are increasing, the incidence of adverse cutaneous reactions due to it is likely to increase proportionately. Imatinib induced Stevens-Johnson syndrome being a life threatening reaction, the physician should keep a high index of suspicion for early diagnosis, prompt withdrawal of the drug and institution of appropriate therapy.
| 1. |
Valeyrie L, Bastuji-Garin S, Revuz J, Bachot N, Wechsler J, Berthaud P, et al. Adverse reactions to imatinib (STI571) in Philadelphia chromosome-positive leukemias: A prospective study of 54 patients. J Am Acad Dermatol 2003;48:201-6.
[Google Scholar]
|
| 2. |
Brouard MC, Prins C, Mach-Pascual S, Saurat JH. Acute generalized exanthematous pustulosis associated with STI571 in a patient with chronic myeloid leukemia. Dermatology 2001;203:57-9.
[Google Scholar]
|
| 3. |
Ena P, Chiarolini F, Siddi GM, Cossu A. Oral lichenoid eruption secondary to imatinib (Glivec). Dermatolog Treat 2004;15:253-5.
[Google Scholar]
|
| 4. |
Hamm M, Touraud JP, Mannone L, Klisnick J, Ponnelle T, Lambert D. Imatinib-induced purpuric vasculitis. Ann Dermatol Venereol 2003;130:765-7.
[Google Scholar]
|
| 5. |
Clark SH, Duvic M, Prieto VG. Mycosis fungoides-like reaction in a patient treated with Gleevec. J Cutan Pathol 2003;30:279-81.
[Google Scholar]
|
| 6. |
Schaich M, Schakel K, Illmer T, Ehninger G, Bornhauser M. Severe epidermal necrolysis after treatment with imatinib and consecutive allogeneic hematopoietic stem cell transplantation. Ann Hematol 2003;82:303-4.
[Google Scholar]
|
| 7. |
Tsao AS, Kantarjian H, Cortes J, O'Brien S, Talpaz M. Imatinib mesylate causes hypopigmentation in the skin. Cancer 2003;98:2483-7.
[Google Scholar]
|
| 8. |
Drummond A, Micallef-Eynaud P, Douglas WS, Hay I, Holyoake TL, Drummond MW. A spectrum of skin reactions caused by the tyrosine kinase inhibitor imatinib mesylate (STI 571, Glivec). Br J Haematol 2003;120:911-3.
[Google Scholar]
|
| 9. |
Banka N, Aljurf M, Hamadah I. Imatinib (STI-571)-induced exfoliative dermatitis in a Saudi patient with deck chair sign. Dermatology 2003;207:329-30.
[Google Scholar]
|
| 10. |
Sanchez-Gonzalez B, Pascual-Ramirez JC, Fernandez-Abellan P, Belinchon-Romero I, Rivas C, Vegara-Aguilera G. Severe skin reaction to imatinib in a case of Philadelphia-positive acute lymphoblastic leukemia. Blood 2003;101:2446.
[Google Scholar]
|
| 11. |
Hsiao LT, Chung HM, Lin JT, Chiou TJ, Liu JH, Fan FS, et al. Stevens-Johnson syndrome after treatment with STI571: a case report. Br J Haematol 2002;117:620-2.
[Google Scholar]
|
| 12. |
Rule SA, O'Brien SG, Crossman LC. Managing cutaneous reactions to imatinib therapy. Blood 2002;100:3434-5.
[Google Scholar]
|
| 13. |
Vidal D, Puig L, Sureda A, Alomar A. STI571-Induced Stevens-Johnson syndrome. Br J Haematol 2002;119:274-5.
[Google Scholar]
|
| 14. |
Brouard M, Saurat JH. Cutaneous reactions to STI571. N Engl J Med 2001;345:618-9.
[Google Scholar]
|
Fulltext Views
2,271
PDF downloads
1,235





