Translate this page into:
Immunofluorescence in dermatology
Correspondence Address:
Seema Chhabra
Room No. 29, 4th Floor, Research Block-A, PGIMER, Sector 12, Chandigarh
India
| How to cite this article: Chhabra S, Minz RW, Saikia B. Immunofluorescence in dermatology. Indian J Dermatol Venereol Leprol 2012;78:677-691 |
Abstract
Direct immunofluorescence (DIF) and indirect immunofluorescence (IIF) tests on skin biopsy are being done mostly in academic teaching hospitals. These tests provide a useful diagnostic aid to dermatologists. Immunohistology and serology can, in conjunction with histology, provide considerable help in delineation and diagnosis of various skin disorders as well as systemic diseases with skin involvement, e.g. systemic lupus erythematosus. Immunofluorescence (IF) studies have now become an invaluable supplement to clinical and histological examination in a variety of dermatological diseases. These skin diseases now include not only bullous and connective tissue disorders, vasculitides, and conditions such as lichen planus, but also the scaling dermatoses, notably psoriasis. In this review article, we share our experience of providing such a diagnostic facility for more than 30 years in a large tertiary care health center in North India and also help to outline the conditions, which can be diagnosed confidently, and others where IF can help in confirming a diagnosis or the immune component of the disease. The article also deals with handling of skin biopsy specimens and interpretation of biopsy findings on DIF and IIF examination.Introduction
Immunofluorescence (IF) microscopy is a well-established technique used for the detection of a wide variety of antigens in tissues or on cells in suspension. This review article deals with extensions of and refinements in the utilization of immunopathology of skin diseases. The objective is to try to maintain and increase the value of immunodermatology in the diagnosis, prognosis, and management of dermatological diseases with immunologic hallmarks.
Coons developed IF in the 1940s with the blue fluorescing compound, β-anthracene. [1] Diagnostic immunopathology in dermatology started in 1963 with the description of the lupus band test (LBT), i.e. deposits of immunoglobulins and complement at the dermo-epidermal junction. [2] Cormane et al. subsequently noted immune deposits in clinically normal skin of patients of systemic lupus erythematosus (SLE), but not in that of discoid LE. [3] In 1964, Beutner and Jordon used the indirect IF technique to demonstrate antibodies in the sera of pemphigus patients. [4] Since that time, the use of IF has grown to be an integral part of a dermatopathology laboratory. The value of IF in diagnostic studies was apparent soon after its introduction and has become routine in the diagnosis of immunologically mediated diseases of the kidney and skin and the demonstration of circulating antibodies such as autoantibodies.
Immunodermatological Methods
Four types of studies are frequently used in immunodermatology, direct and indirect IF, complement fixation, and immunoelectron microscopy. [5]
- Direct immunofluorescence (DIF): DIF is a one step procedure that involves application of fluoresceinated antibodies to a frozen section of the skin. This test determines the deposition of immunoreactants in the patient′s tissue.
- Indirect immunofluorescence (IIF): In IIF, normal whole tissue is the substrate (usually monkey esophagus). This method requires two incubations. The patient′s serum is layered on the substrate followed by application of fluoresceinated antibodies. These studies detect circulating antibodies in the serum. An important advantage of the indirect method is its increased sensitivity (10-15 times). A modified IIF technique using the patient′s own skin as a substrate known as immunomapping (antigen mapping) is used to determine the exact site of cleavage or abnormalities in the distribution of mutated structural proteins (normal, reduced, or lack of expression) in various forms of hereditary epidermolysis bullosa (EB).
- Complement fixation: This is another type of IIF. After the patient′s serum is layered on the substrate, a source of complement is added. Fluoresceinated anticomplement antibodies are then used to detect the presence of complement in the tissue. This test can detect small quantities of complement fixing antibodies.
- Immunoelectron microscopy (IEM): It can be performed in an analogous fashion to detect DIF or IIF. Instead of fluoresceinated antibodies, the antibodies are labeled with an enzyme, such as horseradish peroxidase or a heavy metal, such as colloidal gold. This test provides subcellular or ultrastructural localization of immunoreactants. This technique may be helpful in the differential diagnosis of subtypes of hereditary EB, where antigen mapping is not significant.
There are several advantages of IF over immunoelectron microscopy. IF is a technically simpler and shorter procedure than IEM, and it is more quantitative and reliable. It is also less costly in terms of technician time and reagents; however, tests are less permanent than those made with peroxidase staining. Another major difference is that the biopsy requires specialized cutting of thin sections for immunoelectron microscopy.
Indications for If Studies of Skin Diseases
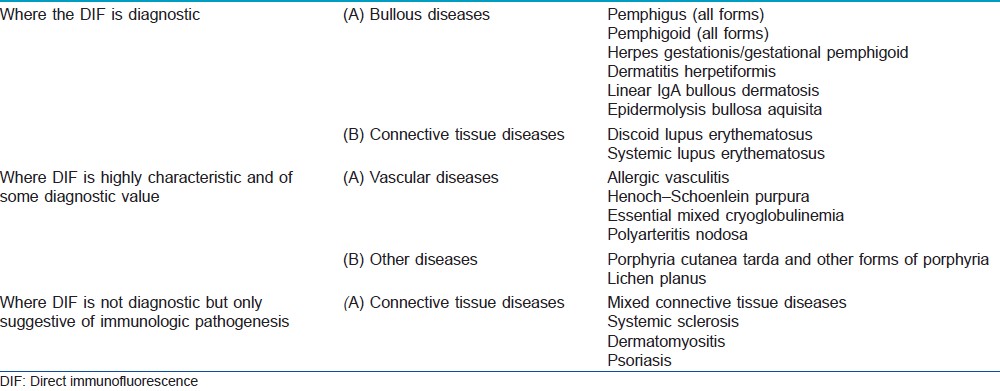
The immunologic findings may be disease specific and diagnostic, they may be characteristic and of some diagnostic value, or they may be of limited diagnostic importance, but their detection may shed some light on the possible participation of immune phenomenon in the pathogenesis of the diseases [Table - 1].
Technical Considerations in ′If′ Studies of Skin Diseases
Types of specimens
Two types of specimens are usually examined by IF techniques. [6]
Skin biopsy specimens: Biopsy specimens are examined for in vivo deposits of serum proteins, usually by the DIF staining method, so examination of skin biopsies is called "immunohistologic study".
Serum samples: About 3 ml of blood without anticoagulants are collected, and the serum is separated from the clotted blood. Clotted blood samples should not be frozen as this causes massive hemolysis. Serum samples are tested for circulating autoantibodies by IIF, so serum studies are referred to as "immunofluorescent serology".
For diagnosis of hereditary EB, about 2-5 ml of ethylenediaminetetraacetic acid (EDTA) blood is needed for mutation analysis.
For any "IF" method, direct or indirect, basically, a section of tissue sample or cellular smear fixed on a glass slide, known as "substrate", is used. In addition, a "conjugate", i.e. an antiserum (e.g. against immunoglobulins, complement components, and fibrinogen) that is covalently linked to a fluorochrome, is required. The fluorochromes used commonly are fluorescein isothiocyanate (FITC), tetramethylrhodamine isothiocyanate, or phycoerythrin.
Skin biopsy specimens
About 3-6 mm punch biopsies are generally adequate.
-
Site of skin biopsy
-
Bullous diseases
Skin biopsy is taken from the unblistered perilesional (normal looking skin) area of the fresh lesion since in older lesions the basement membrane may be completely destroyed, resulting in a negative IF test. It is advantageous to include parts of a fresh blister to get information about the level of split formation. If the biopsy is taken only from the lesional skin, IF again will be negative as in vivo-bound autoantibodies may have been removed by the inflammation. For lesions on mucous membranes, biopsy for immunologic studies should be taken from the periphery of a fresh lesion, since the epithelium in old lesions is usually extensively eroded and therefore the immunofluorescent reaction cannot be detected. [7] Extreme degree of precaution is highly recommended while taking a punch biopsy for hereditary EB as the skin in these patients is extremely fragile and the biopsy procedure itself leads to dermo-epidermal separation causing retention of epidermis within the punch instrument during the procedure. [8]
-
Dermatitis herpetiformis
The standard practice is to obtain biopsy specimens from normal-appearing perilesional skin as inflammation in lesional skin degrades the immune reactants and is usually falsely negative for the diagnostic granular pattern. [9]
-
Lupus erythematosus
Biopsies should be taken from both lesional and apparently normal skin in sun-exposed areas. As a general rule, sun-exposed lesional skin should be used to substantiate an initial diagnosis of LE so as to avoid the problem of false-negative results due to decreased sensitivity in sun-protected areas. [10],[11] In discoid lupus erythematosus (DLE), biopsy should be taken exclusively from lesional skin as uninvolved skin is usually negative.
-
Vasculitis
Very fresh lesions are biopsied as antibody reaction is more prominent. As lesions get older, the IgA deposits get degraded and cleared. In polyarteritis nodosa, deep skin biopsies are needed as IF staining is evident only in some deep arteries in subcutis.
-
Porphyria
Biopsies should be taken from the dorsum of hand lesions as well as normal skin.
-
Lichen planus
Biopsy is taken from inflamed mucosa and/or skin. For clinically normal appearing skin, it is advised to take biopsy from the inner aspect of the upper arm as this part is not exposed to the sun and nonspecific fluorescence does not interfere with the interpretation. It has cosmetic advantage also because the scars are not so obvious. [12]
-
Bullous diseases
-
Processing and handling of skin biopsy specimens
Biopsy specimens taken as indicated above should be either quick-frozen or placed in Michel′s transport medium for later quick freezing. [13] This medium can be used in the combined use of DIF and pre-embedding IEM. [14] Quick freezing is the most widely used method for handling skin biopsy specimens for IF testing. At our institute, we receive all the biopsies in Michel′s transport medium. The samples can be stored for up to 1 month in the refrigerator at 4 °C in this medium (never freeze in Michel′s medium). This transport medium permits shipment of unfrozen skin biopsies at ambient temperatures. Quick freezing and Michel′s medium transport methods cannot be mixed, i.e. frozen tissue cannot be placed in this solution. The results of studies of tissues processed by quick freezing and of those kept in the transport solution are largely comparable. The final pH of the transport medium should be 7.0-7.2. Medium below pH 6.5 or above pH 7.5 gives unsatisfactory results. [15] The shelf life of the medium is over 6 months. Biopsies obtained in Michel′s medium containing a saturated solution of ammonium sulfate in buffer at room temperature should be stored at 4 °C until cut. These biopsies should be washed thrice in phosphate-buffered saline (PBS, pH 7.2, 0.1 M) for 15 min each time before sectioning, otherwise, ammonium sulfate crystals may form, resulting in poor morphology. The standard method in our laboratory is to freeze the specimen in cryostat. Many of the difficulties in IF are related to suboptimal freezing or subsequent storage. For the frozen section, properly oriented tissue is embedded in the OCT (optimal cutting temperature) compound, a water-soluble resin, on a cooled metal chunk in the cryostat and 4-5 μm sections are cut (minimum 10 sections). Section thickness is very important because of its influence on nonspecific staining. Two sections are layered on each slide. The slides are then air dried and stored at −20 °C until they are stained. [16]
-
DIF staining protocol
For the DIF test, the substrates are cryocut sections from patient′s skin or mucous membranes. Cryocut sections must be dry before staining or they will detach and wash away. For staining, sections are brought to room temperature and washed in PBS to remove unbound serum proteins which can significantly contribute to background staining. Optimally diluted FITC-labeled monospecific immunoglobulins (IgG, IgA, and IgM), C 3 , C 5b-9 and fibrin are layered onto the section while it is still moist and incubated at 37 °C for 45 min to 1 h. [16] Then, the sections are washed in PBS thrice and mounted in buffered glycerin. Buffered glycerin at neutral pH is the simplest and most commonly used mountant for IF slides. Rapid fading of fluorescence after excitation is one of the most serious disadvantages of IF techniques, which limits the re-examination of slides for further interpretation. The preservation of fluorescence in DIF-positive slides using mounting media with an antifading reagent is possible for 2 years at room temperature; however, in daily practice, storage for longer than 11 months prevents a reliable diagnosis. [17] Therefore, photography should always be used to document the results.
For the diagnosis of infectious agents, DIF is usually performed on smears prepared from skin (herpes simplex virus, HSV), from genitourinary tract (Chlamydia trachomatis, CT) or from sputum or bronchoalveolar lavage (Pneumocystis carinii, PC). These causative organisms are detected by staining the smears with monoclonal antibody conjugates directed against specific antigens of the respective pathogenic organism. [18]
Practical hints
Skin biopsy cannot be kept beyond 1 month in Michel′s fluid at 4-8 °C. Unlike kidney biopsies, uncut skin biopsies cannot be kept in a frozen state without fluid at -80 °C for any sufficient length of time as they become brittle and fracture easily and lose their orientation during cutting. Sometimes fat content of the dermis can cause difficulty in forming a frozen block with OCT. In such a situation, freezing the skin biopsy in a drop of distilled water facilitates cutting of frozen sections. Once frozen sections of skin biopsies are cut and layered onto the slides, they can be stored indefinitely in deep freezers. During this storage period, thawing of the sections should be absolutely avoided as thawing makes the slides unfit for DIF reporting. Thus, practicing this technique can be extremely difficult in our country where an uninterrupted electrical supply is an improbable proposition in the best hospitals. Before starting the staining procedure, the sections have to be thawed and air dried for at least 2 h at room temperature to prevent floating away of the sections while staining. Freshly stained biopsy should be stored away from light at 4-8 °C and be reported on the same day to prevent quenching of fluorescence. Ideally, digital recording of findings should be maintained.
Serum samples
-
Handling of sera for indirect tests
All sera should be refrigerated until tests are performed. During frozen storage for many months or years, antibody levels decline gradually. Repeated freezing and thawing should be avoided, since this causes a rapid loss of antibody activity. In general, one or two freeze-thaw cycles can be tolerated, but five or more can cause almost complete loss of activity. Positive and negative control sera must be frozen in aliquots of a size adequate for single experiment. For each experiment, these single aliquots should be thawed and diluted to a predetermined concentration. EDTA blood should not be frozen and if this cannot be assured, it is recommended to extract DNA and then store it at -80 °C.
-
Selection of substrates (normal tissue antigens) for IIF microscopy
IIF tests of patient′s sera entail the use of tissue antigens in the form of frozen sections or cell imprints (commercially available or prepared in the own laboratory). Monkey esophagus antigen is the recommended substrate for pemphigus and pemphigoid antibody tests as well as for herpes gestationis factor tests. [19] Guinea pig lip and esophagus, rabbit lip and esophagus, and normal human skin (NHS) have also been tested with satisfactory results. [20] Rat urinary bladder epithelium is used for the diagnosis of paraneoplastic pemphigus. To help separate acquired autoimmune subepidermal bullous disorders where circulating autoantibodies are directed against different components of the basement membrane, human salt-split skin biopsies are used as an excellent substrate. For this purpose, the skin is artificially split through the lamina lucida by incubation overnight in 1 M sodium chloride, so that the lamina lucida remains on the epidermal side and the lamina densa on the dermal side. This technique was first described by Gammon et al. and should preferably be performed on a biopsy from non-sun-exposed skin of patients. [21] For antinuclear antibody (ANA) testing, initially different substrates such as tissue sections, desquamated cells, chicken erythrocytes, and HeLa cells were tried, but later on tissue sections using rat liver or a composite multiblock substrate (mouse stomach, rat liver, and kidney) became the standard substrate. [22] In 1975, HEp-2 cells were introduced which have further increased the sensitivity of the test. These are the cultured monolayered cell preparations of laryngeal squamous cell carcinoma and are available commercially prefixed on glass slides. The majority of laboratories around the world, including ours, are now using HEp-2 cell substrates. Tests for anti-DNA antibodies employ Crithidia luciliae as a substrate. It is a nonpathogenic protozoan with DNA both in the nucleus and the kinetoplast, which is a modified mitochondrion. Thus, IIF on smears of C. luciliae permit identification of antibodies to native or double-stranded DNA. [23] For anti-neutrophil cytoplasmic antibody (ANCA) testing, commercially prepared neutrophil preparations are available or they can be prepared in the laboratory (in house ethanol fixed neutrophil spots). [24]
-
Handling of substrates for IIF
The reliability of the tests depends on the handling of each of the reagents and skill in reading and interpreting the results. Sections of monkey esophagus should be prepared and used within 10 days to 2 weeks after killing and quick freezing. Tissues and sections need to be stored at -70 °C to -80 °C. If the positive control sera fail to give the expected reactions, another piece of fresh esophagus should be used. Sections should be cut at 4 μm for optimal sensitivity. [25]
-
IIF staining protocol
The substrate is incubated with a series of dilutions of patient serum in PBS (mostly starting with 1:10) for 30 min at room temperature and then washed. If present, circulating autoantibodies will bind to the respective antigens in the substrates and are detected by incubation with FITC-labeled, mouse antihuman IgG and / or IgA. Screening for ANA should be done at dilutions of 1/40, because lower dilutions give too many false positives. Even a dilution of 1/40 gives about 20% of ANA positives with normal sera.
Salt split skin test
The salt split skin test (SST) is further of two types: direct salt split skin test (D-SSST) and indirect salt split skin test (I-SSST). D-SSST is performed on patient′s skin biopsy that is either freshly taken (from clinically normal appearing ′patient′ skin) or on the one that has previously been investigated by routine DIF. This allows determining the deposition of in vivo bound autoantibodies either on the blister roof or on the blister floor or on both sides of the artificial split within the BMZ. A major drawback is the loss of the patient′s skin biopsy sample for reinvestigation with routine DIF. For the I-SSST, a sample of NHS is used as a substrate. After artificially inducing the junctional split, cryocut sections are prepared and then IIF with patient′s serum is carried out. This test is much more sensitive than routine IIF and is very helpful in patients where a biopsy is not available.
Conjugates
Conjugates used in immunodermatology are of two types, monospecific reagents used for direct staining of biopsies, and human anti-whole-IgG conjugates used for the IIF test of sera. They are selected on the basis of their fluorescein/protein (F/P) ratio and antibody (anti-Ig antibody protein) content. A medium range molar F/P ratio of 1.8-2.5 is commonly used. Specificity and high degree of reactivity are the two most important prerequisites of fluorochrome-labeled antiseras or antibodies used in IF studies. Data on specificity and reactivity are supplied by the manufacturer; however, confirmation of specificity and reactivity is the responsibility of the user. The most satisfactory means of determining antibody reactivity and specificity is through the appropriate use of positive and negative controls. Most commercial conjugates are supplied in a lyophilized form and require reconstitution with distilled water, or in diluents supplied by the manufacturer. The undiluted stock should be divided into volumes of 0.10-0.50 ml and stored frozen (−20 °C) until ready to be diluted. The diluted conjugates should be stored at 4 °C and not refrozen, since freezing and thawing can be detrimental to antibody reactivity, particularly at dilute concentrations. A preliminary chessboard titration based on antibody content of the conjugate should be performed before tests are undertaken. These are performed with serial dilutions of a known positive serum and with a series of conjugate dilutions containing known concentration of antibody. [25]
Evaluation of Staining Results
Skin biopsies
Interpretation of findings requires a clear understanding of all of the reaction patterns associated with bullous diseases, connective tissue immune complex diseases, vasculitides, and the reactions in stratum corneum. While reporting skin biopsy, the fluorescent staining should be described under the following headings [16] : (i). Type of immunoreactant: IgG, IgA, IgM, C3, fibrin, C5b-9. (ii) Location of immune deposits: intercellular spaces (ICS) in epidermis/epidermal nuclear staining (ENS) or in vivo ANA/basement membrane zone (BMZ)/subepidermal blood vessels/hair shaft/cytoid (civatte or colloid) bodies. (iii) Extent of staining: Focal or diffuse. (iv) Intensity of staining: Semi-quantitative grading of strength of fluorescence: + to ++++. (v) Pattern of immune complex deposits: granular or linear. The granular pattern is further divided into coarse granules, speckles, threads, and fibrils. The description of all these staining characteristics leads to immunopathological diagnosis.
Key points to remember while reporting
There is often a need to compare relative intensities of staining between positive and background staining in control slides. High background often results from antibodies that are too heavily conjugated with fluorescein; these molecules adhere nonspecifically to tissue because of their negative charge. It may also be the result of partial drying of tissue sections after the staining procedure has begun. In addition to the desired specific staining, the immunopathologist must also be aware of nonspecific staining and autofluorescence. Autofluorescence, due to fluorescence found intrinsically in the tissue (recognized usually by its color which is generally yellow or orange), should be distinguished from the apple green color of fluorescein. Examination of an unstained (control) section can be helpful in judging autofluorescence. Nonspecific staining is fluorochroming due to staining capacity of FITC-labeled proteins. It depends on the affinity of the specific components of the antigenic substrate for nonspecific binding of labeled protein and the concentration of fluorescein in the dilution of conjugate selected for staining. Nonspecific staining is commonly encountered in thicker sections. Sections cut at 6 μm or thicker cannot be used for IF because of high nonspecific staining. Slides should be examined preferably on the day of staining and a detailed record of results as outlined above should be kept on work sheets. It is wise to run positive and negative controls with each biopsy, until one gains confidence in specificity of staining with a given conjugated antibody.
Laboratories
Only qualified laboratories should do such tests; biopsy specimens and blood samples should be sent to the laboratories that perform them with reliable methods, since results of unreliable test procedures may result in misdiagnosis and error in management. It must be kept in mind that immunofluorescent tests when performed by inexperienced workers frequently lead to misinterpretations. To use these methods successfully, the laboratory worker should have about a half year of training in immunology and immunodermatology. Formal programs for certification in immunopathology of the skin should be instituted. In a nutshell, these studies should be performed at large institutions that have the necessary trained personnel, resources, and experience to do the work properly since the prerequisites for the proper performance of these tests are rather demanding.
Diagnostic and Prognostic Applications of Immunofluorescence Studies
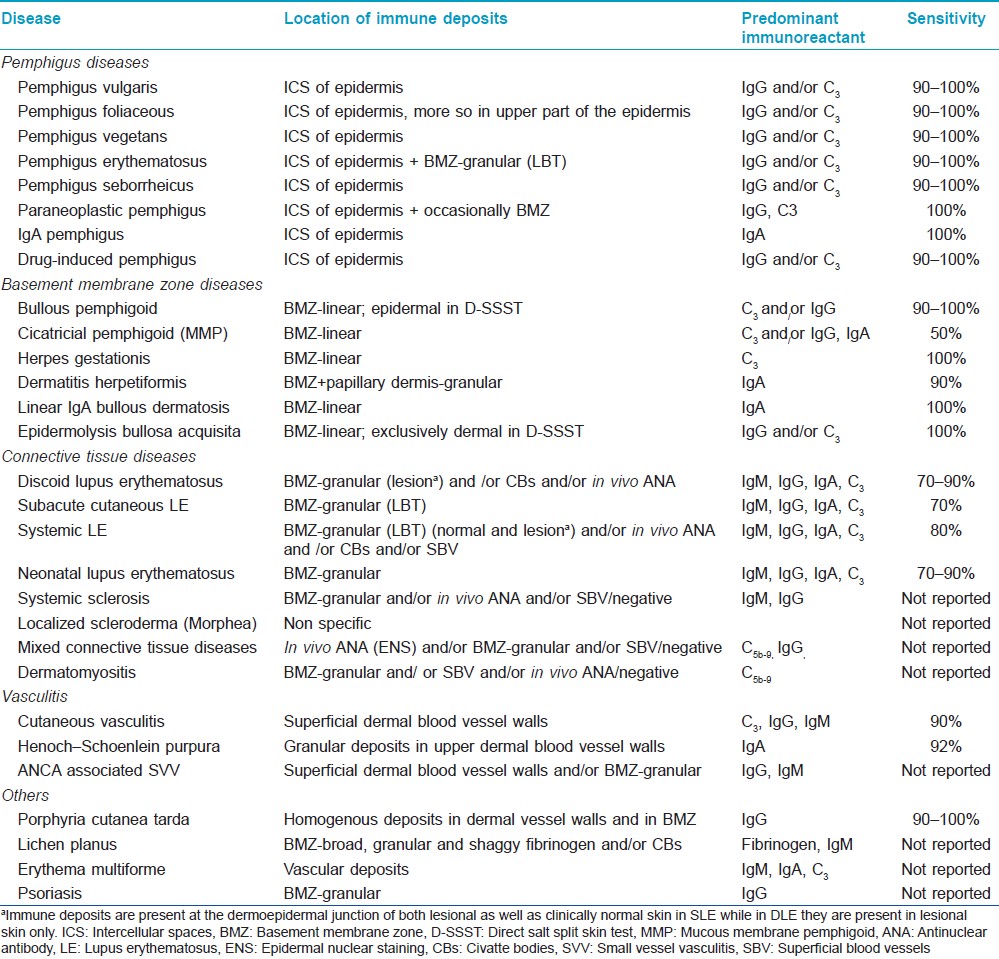
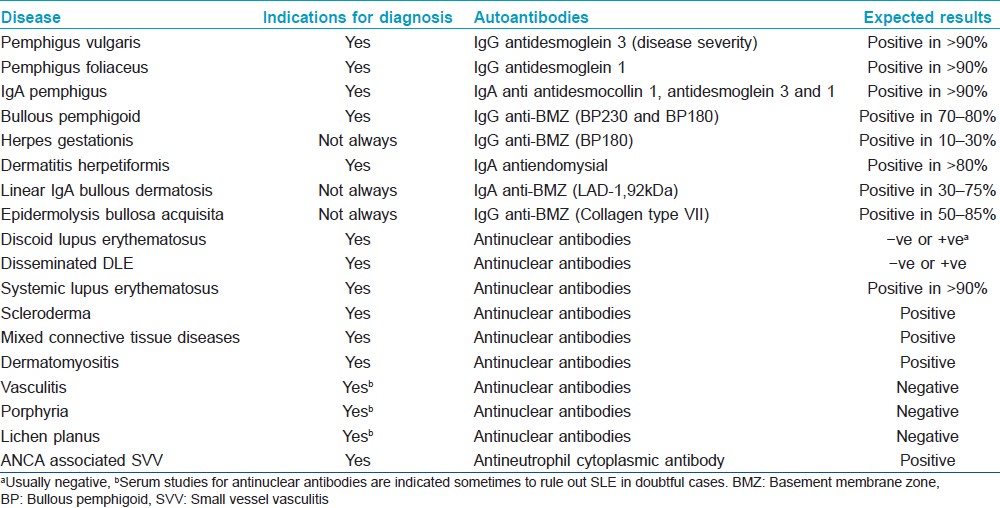
DIF as well as IIF findings for various cutaneous disorders have been summarized in [Table - 2] and [Table - 3].
Pemphigus
Pemphigus vulgaris (PV) is a common autoimmune vesiculobullous disorder. DIF of perilesional skin in pemphigus shows linear/granular deposition of immunoglobulins (Igs) and C3 in the epidermal ICS [Figure - 1]. This pattern is described as the "chicken wire" pattern. All forms of pemphigus produce the same pattern, though there is some tendency for staining to be more superficial within the epidermis in PF than in PV. In pemphigus erythematosus, a mixed pattern is seen, i.e. ICS positivity with a band of immune deposits along the dermo-epidermal junction (DEJ). IgG is present in nearly 100% of patients whereas C3 is present in 50-100% of cases. The intensity of DIF coincides fairly well with clinical activity, but a few patients may continue to have positive DIF despite clinical inactivity of pemphigus. [26],[27] In patients who relapse, DIF tends to remain positive and the complement deposition may show an increase or may reappear. It is, thus, a better indicator of an imminent relapse. Sethi et al. highlighted that a positive DIF is perhaps not a good prognostic marker for disease activity as it continued to remain positive even in patients who were clinically inactive. [26] The pemphigus-specific immunofluorescence pattern seen in the skin has also been demonstrated in the outer root sheaths of plucked hair follicles in 85-100% cases of PV. [28],[29],[30],[31],[32] The role of DIF techniques on Tzanck smear samples has also been evaluated for the diagnosis of PV, and the results were found to be comparable with respective skin biopsies in the same set of patients. [33] Thus, Tzanck smears can serve as an alternative to surgical biopsies especially in rural areas lacking proper histological equipment. IgA pemphigus is characterized by IgA deposition in the ICS throughout the epidermis with increased intensity in the upper layers. IIF studies on appropriate epithelial substrates detect circulating IgG autoantibodies, which produce the characteristic ICS pattern of fluorescence. The presence of these autoantibodies is abnormal at any titre and generally correlate with disease activity. ELISA (desmoglein 1 and 3) is a quick, simple, sensitive, and efficient tool for confirming the diagnosis as well as predicting relapse. [34],[35],[36]
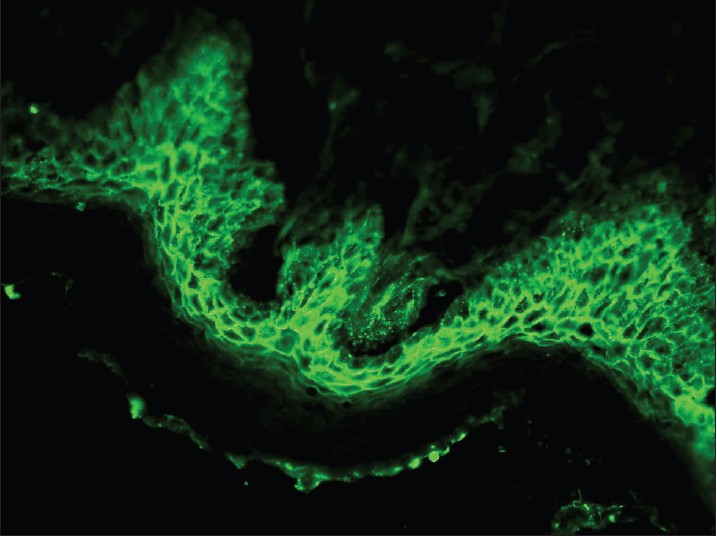 |
| Figure 1: DIF photomicrograph of a case of PV showing IgG reactive granular (+++) deposits at the intercellular spaces in the epidermis (×400) |
Bullous pemphigoid
Bullous pemphigoid (BP) is subepidermal bullous disease of unknown cause. Major antigens in BP are two keratinocyte hemidesmosomal proteins, an intracellular 230 kDa protein (BP230) and 180 kDa protein (BP180). DIF of unblistered, perilesional skin from the edge of a fresh blister is diagnostically sensitive and typically demonstrates linear BMZ fluorescence in nearly 100% of patients [Figure - 2]. C 3 and IgG, or C 3 alone are the predominant immunoreactants. A linear BMZ pattern can be seen in several disparate clinical disorders, including epidermolysis bullosa acquisita (EBA) and gestational pemphigoid. [37] In BP and gestational pemphigoid, linear IgG is localized to the roof (epidermal side) of the split whereas in EBA, linear deposition of immunoreactants is seen along the dermal side of the split. [38],[39],[40] IIF detects circulating IgG anti-BMZ autoantibodies in about 50% of the patients with pemphigoid, but the sensitivity increases to 80% if normal human salt-split skin is used as a substrate. The combined ELISAs based on BP180 and BP230 fragments are very useful in establishing the diagnosis of BP and detect IgG autoantibodies in 96% of all BP sera and thus can be used as adjunctive diagnostic aid. [41]
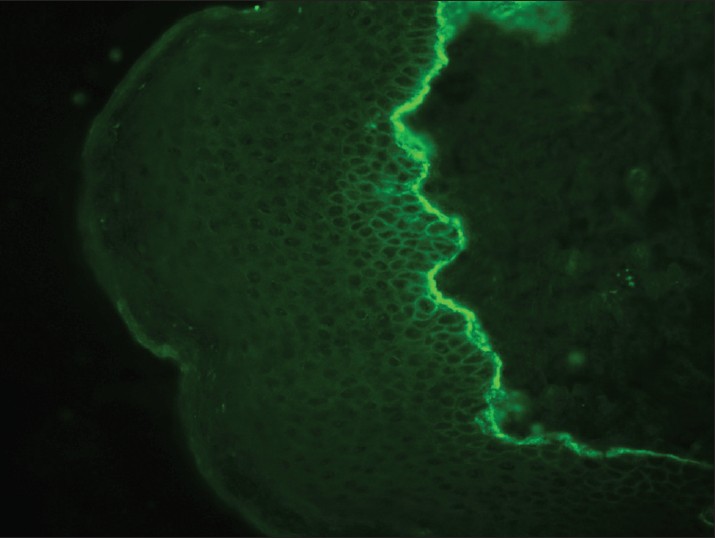 |
| Figure 2: DIF photomicrograph of a case of BP showing IgG reactive linear (+++) deposits at the dermo-epidermal junction (×400) |
Gestational pemphigoid
It develops during or soon after pregnancy. DIF from perilesional skin typically shows linear C 3 BMZ deposits in 100% of cases. IgG may be seen in 30% cases. [42]
Epidermolysis bullosa acquisita
It is an acquired subepidermal blistering disease characterized by the presence of autoantibodies to type VII collagen in BMZ. DIF of direct salt-split skin biopsy tissue shows linear deposits of immunoreactants at the dermal, or floor aspect of the induced blister. On IIFM, only about 40% of EBA patients have demonstrable antibodies. [43],[44]
Dermatitis herpetiformis
Dermatitis herpetiformis (DH) is a pruritic subepidermal bullous disease associated with gluten sensitive enteropathy. The direct biopsy should be from perilesional skin because a lesional skin biopsy may be negative. [9] DIF detects granular IgA deposits in the tips of dermal papillae in 80-100% of patients [Figure - 3]. IIF shows antiendomysial IgA autoantibodies in 80% of patients, in subepithelial muscularis mucosa of monkey esophagus as a substrate.
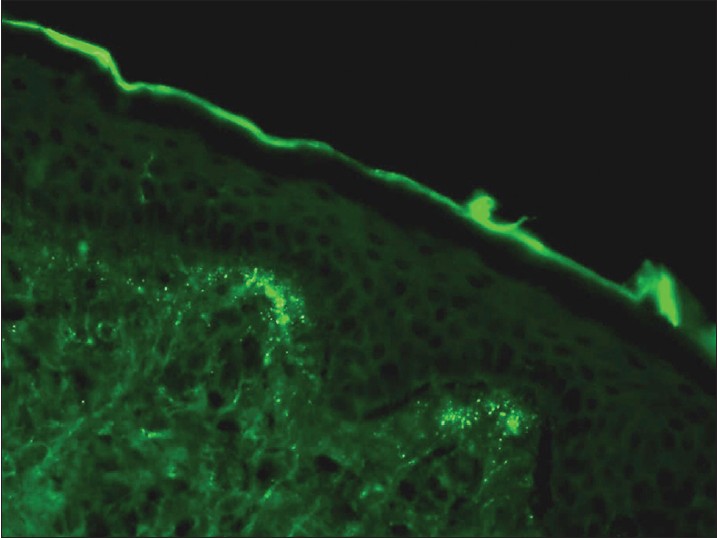 |
| Figure 3: DIF photomicrograph of a case of DH showing IgA reactive granular (+++) deposits at the tips of dermal papillae (×400) |
Linear IgA dermatosis
Linear IgA dermatosis is a distinct clinical entity also known as chronic bullous dermatosis of childhood. In nearly 100% cases, DIF demonstrates intense linear IgA deposits along the BMZ, but none in dermal papillae. Weaker staining for IgG, IgM, and/or C 3 is also found in about 20% of cases. This pattern of staining is not entirely specific for linear IgA dermatosis as some cases of EBA that are caused by IgA anti-collagen VII antibodies produce a similar pattern on DIF. Linear IgA at the BMZ also occurs in 20% of mucous membrane pemphigoid cases. In these diseases, the IgA is often accompanied by other immunoreactants at the BMZ, but this can also occur in linear IgA disease, and there is some overlap among the three conditions. Linear or granular IgA can also be observed at the BMZ in lupus. In SSST, linear staining can be seen on roof or floor. On IIF, circulating IgA anti-BMZ autoantibodies can be detected in about 10-30% cases. [45]
Lupus erythematosus
With DIF, one sees characteristic coarse, granular, continuous [Figure - 4] deposits of Igs and complement along the DEJ (LBT). The reported sensitivity of DIF examination for lupus ranges from 58% to 93%. [46] Although characteristic, this reaction pattern can be seen in other disorders such as mixed connective tissue disease, dermatomyositis, scleroderma, drug eruptions, and vasculitis. Therefore, it is mandatory to correlate DIF results with both clinical presentation and histopathological findings. [47] Up to 95% of individuals with DLE show LBT. However, positive DIF findings in DLE are confined to lesional skin, whereas skin with or without lesions can give positive DIF findings in SLE. [48] Cytoid bodies (CBs) can be seen in DLE. Although certainly not specific for lupus, the presence of CBs might increase the index of suspicion for LE in difficult cases. In vivo ANA or ENS [Figure - 5] has also been observed in connective tissue disorders mainly, with SLE being the commonest disorder. [49],[50] It has also been noted that prolonged formalin exposure of skin biopsies artifactually allows fluorescein-labeled antibodies to produce the ENS pattern which can be mistaken for in vivo ANA reactions of LE. Therefore, while reporting the ENS pattern, one should specifically rule out the possibility of inadvertent exposure of skin biopsy to formalin. [51] IIF studies of the patients′ sera reveal high incidence of ANAs. Different patterns of nuclear fluorescence, i.e. diffuse, nucleolar, centromeric, and speckled (fine or coarse) can be easily appreciated on HEp-2 cells [Figure - 6] and [Figure - 7]. Various ELISAs directed against different nuclear antigens are commercially available. In addition, immunoblots [Figure - 8] can also be used to determine antinuclear antibodies directed against various nuclear antigens in a single experiment.
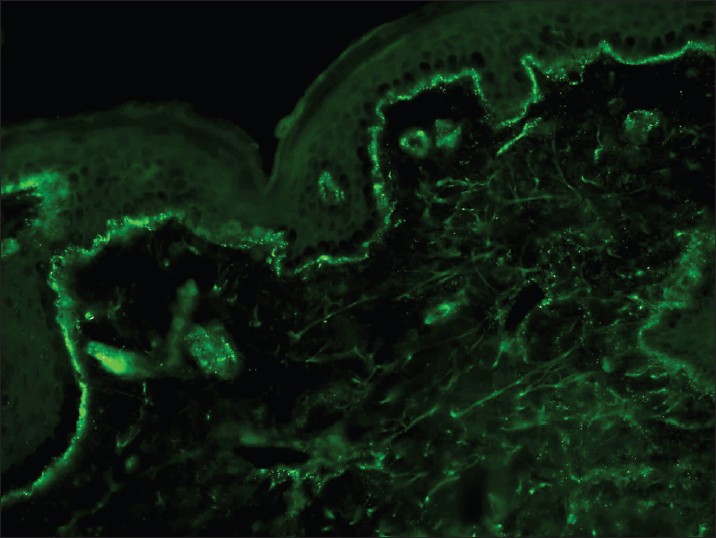 |
| Figure 4: DIF photomicrograph of a case of SLE showing IgG reactive granular (+++) deposits at the dermo-epidermal junction and superficial dermal blood vessels (×400) |
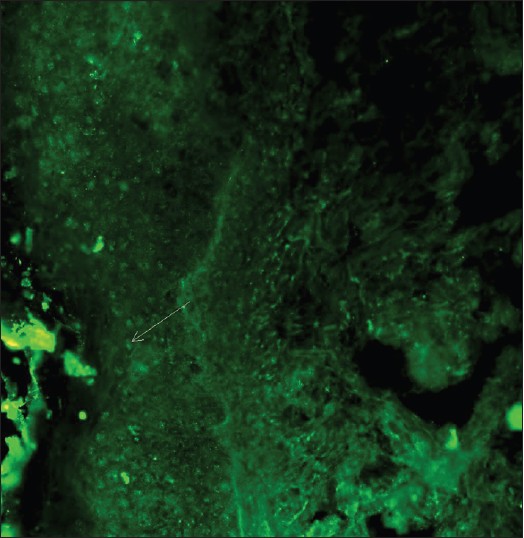 |
| Figure 5: DIF photomicrograph of a case of SLE showing IgG reactive (+++) epidermal nuclear staining or in vivo ANA (×400) |
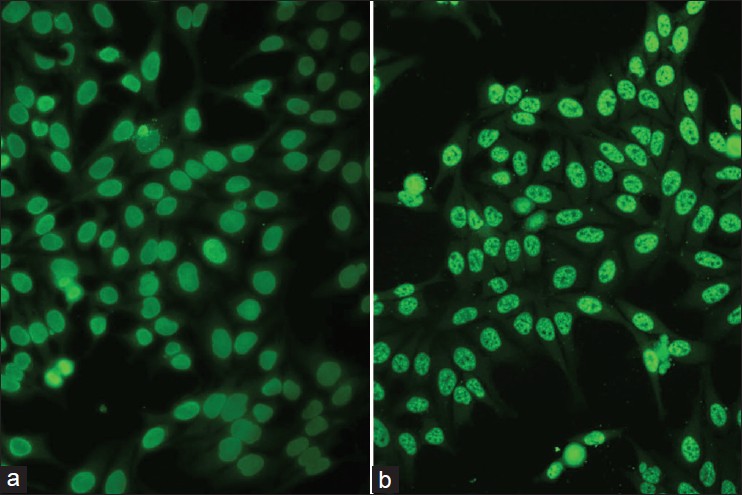 |
| Figure 6: IIF photomicrograph of Hep2 cell lines showing (a) diffuse homogenous pattern and (b) speckled pattern of nuclear fluorescence (×400) |
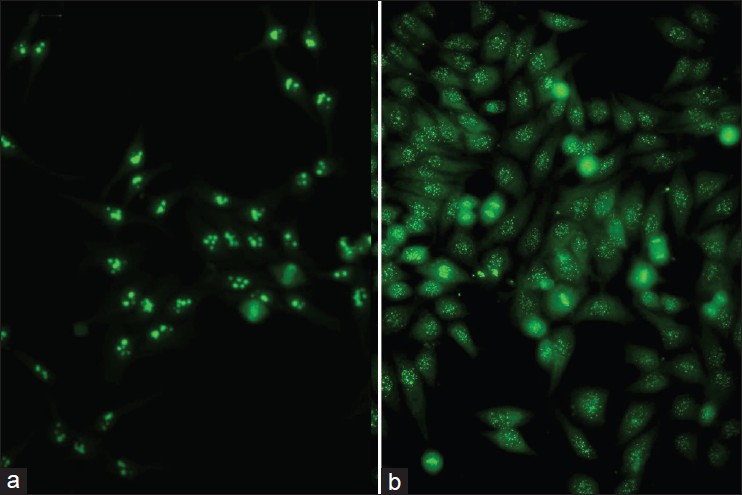 |
| Figure 7: IIF photomicrograph of Hep2 cell lines showing (a) nucleolar pattern and (b) centromeric pattern of nuclear fluorescence (×400) |
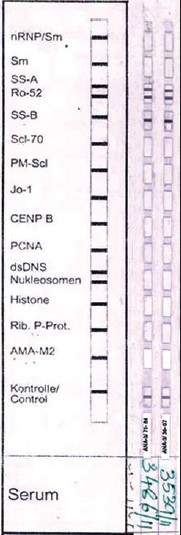 |
| Figure 8: Immunoblot with 15 antigens showing positivity for SS-A and SS-B. |
Scleroderma, dermatomyositis, and MCTDs
DIF of lesional skin from patients with dermatomyositis and MCTDs may yield similar, if not indistinguishable, fluorescence results as in DLE. In addition, nuclear keratinocyte decoration (in vivo ANA) with IgG and C 5b-9 may be seen in MCTDs. [52] In diffuse scleroderma, the frequency of positive LBT consisting of IgG or IgM class and/or in vivo ANA varies from 0 to 60%. [53]
Vasculitis including anti-neutrophil cytoplasmic antibody associated small vessel vasculitides and porphyria
In vasculitis, DIF of skin biopsies taken from very fresh lesions display vascular deposits of IgM, C 3 , fibrinogen, and sometimes IgG. A negative result does not exclude vasculitis. Skin biopsy from HSP lesions is characterized by predominant deposition of IgA [Figure - 9] in the walls of upper dermal vessels. [54] Immune deposits can be detected in skin biopsies of patients with Wegener′s granulomatosis (WG) with IgG being the most common immunoreactant deposited in and around subepidermal blood vessels, but occasionally also along the BMZ. [55] These results challenge the current notion that WG is a genuine pauci-immune vasculitis. On IIF of sera, ANCA associated small vessel vasculitides (AASVV) are characterized by the presence of circulating ANCA [Figure - 10] and [Figure - 11]. Various ELISAs directed against various ANCA specificities (including PR3, MPO, lactoferrin, etc.) are routinely available.
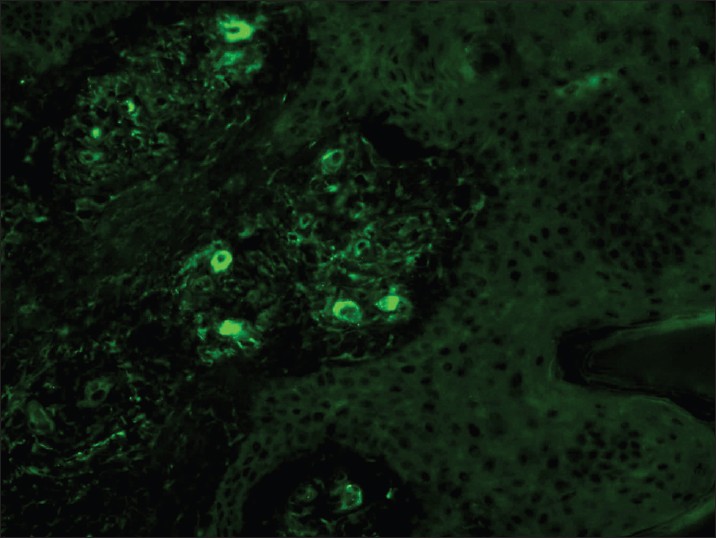 |
| Figure 9: DIF photomicrograph of a case of HSP showing IgA reactive granular (+++) deposits in the blood vessel walls in the upper dermis (×400) |
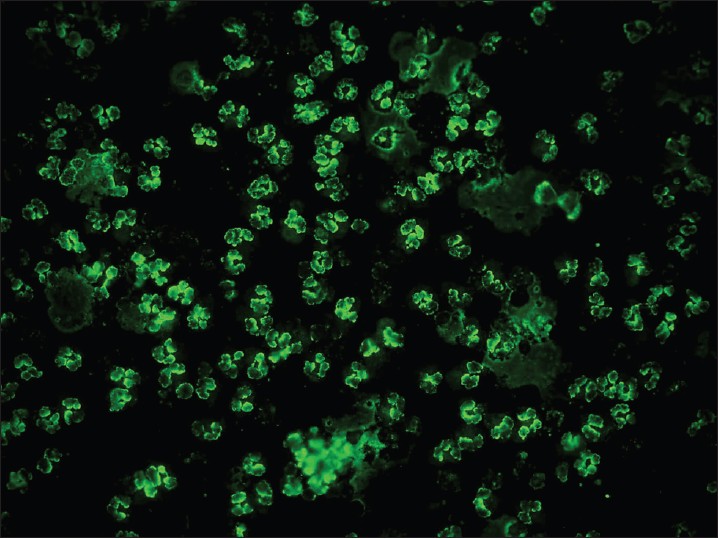 |
| Figure 10: IIF photomicrograph of ethanol fixed neutrophil preparation showing P-ANCA pattern of perinuclear fluorescence (×400) |
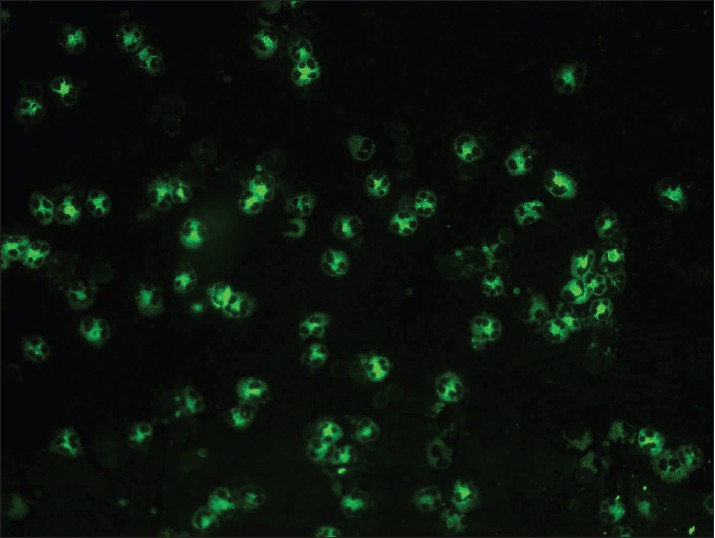 |
| Figure 11: IIF photomicrograph of ethanol fixed neutrophil preparation showing C-ANCA pattern of granular cytoplasmic fluorescence with central lobular accentuation (×400) |
Lichen planus
DIF of lesions of lichen planus (LP) is characterized by large, grouped and globular deposits of immunoglobulins [Figure - 12] and complement, i.e. CBs. Any immunoreactant deposit at CBs plus shaggy fibrinogen deposition, whether alone or combined with other immunoreactants at DEJ, are among the most important findings on DIF favoring the diagnosis of LP. [56] In LP, CBs tend to be more numerous in number as well tend to form clusters or groups of 10 or more in the papillary dermis. This cluster formation on DIF may be useful in distinguishing LP from LE, because, in the latter condition, when CBs are present, they tend to exhibit a more linear arrangement. The positive yield of DIF in LP is in the range of 37-97%. [57],[58]
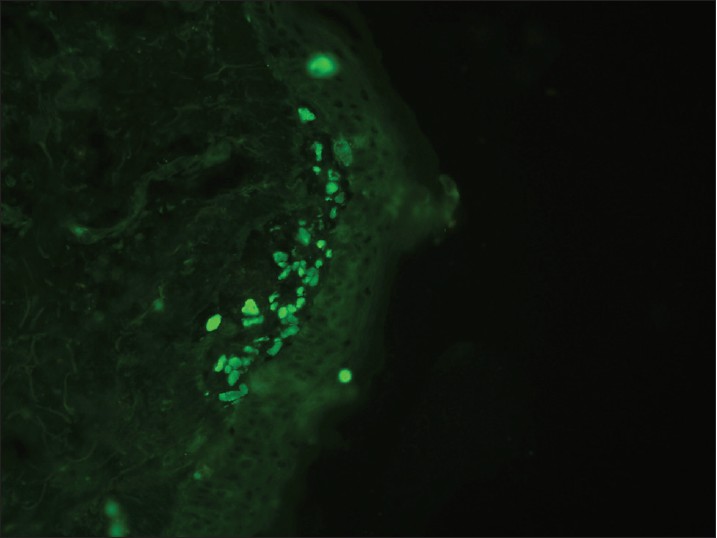 |
| Figure 12: DIF photomicrograph of a case of LP showing IgM reactive large grouped globular (++) deposits (CBs) in the papillary dermis (×400) |
Psoriasis
It has been reported that only 3% biopsy specimens from sun-exposed psoriatic lesions may demonstrate bright continuous bands of granular positivity along the DEJ with IgG, IgM, C3, and fibrinogen. [59]
Cutaneous infectious diseases
In infectious diseases caused by CT, HSV, and PC, the microbial agents can be easily visualized in the smears with DIF and circulating antibodies against these agents are found by IIF. By using the monoclonal antibody conjugates directed against specific antigens of the corresponding microbial agents, DIF highlights cytoplasmic staining in HSV type 1-infected cells, nucleolar staining in HSV type 2-infected cells, elementary bodies in CT-infected samples and clusters of cysts in PC-infected samples thereby making the diagnosis much more easier. [18]
Advantages and Pitfalls of If
IF is a useful clinical aid when it comes to diagnosing autoimmune bullous disorders. Some of the advantages of IF are as follows:
- To assist in the diagnosis of various dermatological disorders, from relatively mild ones such as LP to more lethal ones such as PV.
- To classify various autoimmune bullous diseases which may have a confusingly similar clinical profile.
- To clarify the picture in cases of autoimmune bullous disorders where, clinically, no diagnosis has been made due to the atypical appearance and nonspecific characteristics of the lesions.
- The various circulating autoantibodies that are detected by IIF provide an indirect measure of the activity of the disease and can help in deciding which therapeutic options might be most beneficial to the patient.
- Antigen mapping can help in marking for a particular protein the presence or absence of which can play a role in classifying various forms of hereditary EB.
- The clinical picture of some infections (HSV, Chlamydia) is often muddled and unclear in patients that are immunosuppressed from any cause. In such cases, DIF can provide a rapid and convenient method to achieve a diagnosis.
This technique has numerous pitfalls and difficulties too, which should be borne in mind while using it for skin disease diagnostics. It can be processed only in advanced laboratories that are proficient in the performance and interpretation of these tests. It requires a thoroughly trained team comprising of a pathologist and a technologist in a specialized lab having facilities of cryostat for cutting frozen sections, −20° C/−80 °C deep freezers to store these sections until staining and a fluorescence microscope to report the DIF findings. Skin biopsy is fragile and needs to be transported in Michel′s fluid only. Also it gets fixed on exposure to formalin vapors so extreme care is required in handling the specimen. Dried and/or fixed biopsies are reported unfit for DIF. The fluorescence staining quenches rapidly on exposure to light, more so under the UV light of the fluorescence microscope, resulting in the necessity of fast reporting and documentation of findings using a digital camera. No long-term storage period is ideal for reporting of DIF-stained skin biopsy slides.
Given the importance of IF as outlined above, it is desirable to create facilities for DIF examination of skin biopsies, along with relevant supportive serologic studies in referral government hospitals across the country. This will greatly improve confidence in diagnosis of various cutaneous disorders as already described, and thereby also improve therapy and finally outcome in these conditions.
Acknowledgments
The authors are grateful to Mrs. Shashi Anand (senior technologist) for providing the technical expertise of cutting, storing, and staining skin biopsies for direct immunofluorescence microscopy for the last 30 years and sharing her experience with us.
| 1. |
Coons AH, Creech HJ, Jones RN. Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol Med 1941;47:200.
[Google Scholar]
|
| 2. |
Burnham TK, Neblett TR, Fine G. The application of fluorescent antibody technique to the investigation of lupus erythematosus and various dermatoses. J Invest Dermatol 1963;41:541-56.
[Google Scholar]
|
| 3. |
Beutner E, Jablonska S, Kumar V. Direct Immunofluorescence in lupus erythematosus. In: Beutner E, Chorzelski TP, Beau SF, editors. Immunopathology of skin. New York/Brisbane/Singapore: Wiley Medical Publication; 1987. p. 499-520.
[Google Scholar]
|
| 4. |
Mohan KH, Pai S, Rao R, Sripathi H, Prabhu S. Technique of immunofluorescence and their significance. Indian J Dermatol Venereol Leprol 2008;74:415-9.
[Google Scholar]
|
| 5. |
Black MM, Bhogal BS, Willsteed E. Immunopathological techniques in the diagnosis of bullous disorders. Acta Derm Venereol 1989;69:96-105.
[Google Scholar]
|
| 6. |
Cooperative study. Uses for immunofluorescence tests of skin and sera. Arch Dermatol 1975;111:371-86.
[Google Scholar]
|
| 7. |
Beutner EH, Kumar V, Krasny DA. Defined immunofluorescence: Basic concepts and their application to clinical immunodermatology. In: Beutner EH, Chorzelski TP, Kumar V, editors. Immunopathology of the skin. 3rd edn. New York: John Wileyand Sons; 1987. p. 3-40.
[Google Scholar]
|
| 8. |
Intong LR, Murrell DF. How to take skin biopsies for epidermolysis bullosa. In: Murrell DF, editor. Epidermolysis bullosa part II- diagnosis and management. Vol. 28. Philadelphia: W. B. Saunders Elsevier; 2010. p. 197-200.
[Google Scholar]
|
| 9. |
Sousa L, Bajanca R, Cabral J, Fiadeiro T. Dermatitis Herpetiformis: Should direct immunofluorescence be the only diagnostic criterion? Pediatr Dermatol 2002;19:336-9.
[Google Scholar]
|
| 10. |
Mehta V, Sarda A, Balachandran G. Lupus band test. Indian J Dermatol Venereol Leprol 2010;76:298-300.
[Google Scholar]
|
| 11. |
Kontos AP, Jirsari M, Jacobsen G, Fivenson DP. Immunoglobulin M predominance in cutaneous lupus erythematosus. J Cutan Pathol 2005;32:352-5.
[Google Scholar]
|
| 12. |
Pohla-Gubo G, Becher E, Romani N, Fritsch P, Hintner H. Salt-split test on normal, non sun-exposed skin of patients with auto-immune subepidermal bullous diseases. Dermatology 1994;189 Suppl 1:123.
[Google Scholar]
|
| 13. |
Michel B, Milney Y, David K. Preservation of tissue fixed immunoglobulins in the skin biopsy of patients with lupus erythematosus and bullous pemphigoid. J Invest Dermatol 1972;59:449-52.
[Google Scholar]
|
| 14. |
Vaughn Jones SA, Palmer I, Bhogal BS, Eady RA, Black MM. The use of Michel's transport medium for immunofluorescence and immunoelectron microscopy in autoimmune bullous diseases. J Cutan Pathol 1995;22:365-70.
[Google Scholar]
|
| 15. |
Nisengard RJ, Jablonska S, Chorzelski TP, Beutner EH. Immunofluorescence of biopsy specimens: comparison of methods of transportation. Immunofluorescence studies. Arch Dermatol 1978;114:1329-32.
[Google Scholar]
|
| 16. |
Minz RW, Chhabra S, Singh S, Radotra BD, Kumar B. Direct immunofluorescence of skin biopsy: Perspective of an immunopathologist. Indian J Dermatol Venereol Leprol 2010;76:150-8.
[Google Scholar]
|
| 17. |
Dikicioglu E, Meteoglu I, Okyay P, Culhaci N, Kacar F. The reliability of long term storage of direct immunofluorescent staining slides at room tempreture. J Cutan Pathol 2003;30:430- 6.
[Google Scholar]
|
| 18. |
Pohla-Gubo G, Kraus L, Hintner H. Role of Immunofluorescence microscopy in dermatology. Gital Dermatol Venereol 2011;146:127-42.
[Google Scholar]
|
| 19. |
Kumar V, Beutner EH. Monkey esophagus: A unique antigenic substrate in immunodermatology. In: Beutner EH, Chorzelski TP, Bean SF, editors. Immunopatholgy of skin. 3rded. New York: John Wiley and Sons; 1987. p. 65-94.
[Google Scholar]
|
| 20. |
Bystryn JC, Sabolinski M. Effect of substrate on indirect immunofluorescence tests for intercellular and basement membrane zone antibodies. J Am Acad Dermatol 1986;15:973- 7.
[Google Scholar]
|
| 21. |
Gammon WR, Kowalewski C, Chorzelski TP, Kumar V, Briggaman RA, Beutner EH. Direct Immunofluorescence studies of sodium chloride separated skin in the differential diagnosis of bullous pemphigoid and epidermolysis bullosa acquisita. J Am Acad Dermatol 1990;22:664-70.
[Google Scholar]
|
| 22. |
Kumar Y, Bhatia A, Minz RW. Antinuclear antibodies and their detection methods in diagnosis of connective tissue diseases: A journey revisited. Diagn Pathol 2009;4:1.
[Google Scholar]
|
| 23. |
Vassileva S. Immunofluorescence in dermatology. Int J Dermatol 1993;32:153-61.
[Google Scholar]
|
| 24. |
Minz RW, Chhabra S, Rani L, Singh S, Jindal SK, Sakhuja V. A decade long experience of anti-neutrophil cytoplasmic antibody testing in a tertiary care referral center in North India: Perspective from a developing country. Indian J Pathol Microbiol 2011;54:258-63.
[Google Scholar]
|
| 25. |
Beutner EH, Nisengard RJ, Kumar V. Defined Immunofluorescence: Basic concepts and their application to clinical immunodermatology. In: Beutner EH, Chorzelski TP, Bean SF, eds. Immunopathology of the skin. 2 nd edn. New York: John Wiley and Sons; 1979. p. 29-75.
[Google Scholar]
|
| 26. |
Sethi KJ, Kanwar AJ, Kaur S, Sehgal S. Direct immunofluorescence as a diagnostic and prognostic marker in pemphigus. Indian J Dermatol Venereol Leprol 1992;58:379-83.
[Google Scholar]
|
| 27. |
Ratnam KV, Pang BK. Pemphigus in remission: Value of negative direct immunofluorescence in management. J Am Acad Dermatol 1994;30:547-50.
[Google Scholar]
|
| 28. |
Kumaresan M, Rai R, Sandhya V. Immunofluorescence of the outer root sheath in anagen and telogen hair: An aid to diagnosis in pemphigus. Int J Trichol 2009;1:138-9.
[Google Scholar]
|
| 29. |
Schaerer L, Trüeb RM. Direct immunofluorescence of plucked hair in pemphigus. Arch Dermatol 2003;139:228-9.
[Google Scholar]
|
| 30. |
Rao R, Shenoi SD, Balachandran C. Demonstration of pemphigus specific immunofluorescence pattern by direct immunofluorescence of plucked hair. J Am Acad Dermatol 2008;58:AB85.
[Google Scholar]
|
| 31. |
Daneshpazhooh M, Asgari M, Naraghi ZS, Barzgar MR, Akhyani M, Balighi K, et al. A study on plucked hair as a substrate for direct immunofluorescence in pemphigus vulgaris. J Eur Acad Dermatol Venereol 2009;23:129-31.
[Google Scholar]
|
| 32. |
Kumaresan M, Rai R, Sandhya V. Immunofluorescence of the outer root sheath: An aid to diagnosis in pemphigus. Clin Exp Dermatol 2011;36:298-301.
[Google Scholar]
|
| 33. |
Aithal V, Kini U, Jayaseelan E. Role of direct immunofluorescence on Tzanck smears in pemphigus vulgaris. Diagn Cytopathol 2007;35:403-7.
[Google Scholar]
|
| 34. |
Mortazavi H, Shahdi M, Amirzargar AA, Naraghi ZS, Valikhani M, Daneshpazhooh M, et al. Desmoglein ELISA in the diagnosis of pemphigus and its correlation with the severity of pemphigus vulgaris. Iran J Allergy Asthma Immunol 2009;8:53-6.
[Google Scholar]
|
| 35. |
Kumar B, Arora S, Kumaran MS, Jain R, Dogra S. Study of desmoglein 1 and 3 antibody levels in relation to disease severity in Indian patients with pemphigus. Indian J Dermatol Venereol Leprol 2006;72:203-6.
[Google Scholar]
|
| 36. |
Sharma VK, Prasad HR, Khandpur S, Kumar A. Evaluation of demoglein enzyme-linked immunosorbent assay (ELISA) in Indian patients with pemphigus vulgaris. Int J Dermatol 2006;45:518-22.
[Google Scholar]
|
| 37. |
Mahmood T, Haroon TS. Patterns of direct immunofluorescence in sub-epidermal autoimmune bullous diseases of skin in Lahore, Pakistan. J Pak Assoc Dermatol 2003;13:67-71.
[Google Scholar]
|
| 38. |
Domloge-Hultsch N, Bisalbutra P, Gammon WR, Yancey KB. Direct Immunofluorescence microscopy of 1 mol/L sodium chloride treated patient skin. J Am Acad Dermatol 1991;24:946-51.
[Google Scholar]
|
| 39. |
Barnadas MA, Gonzalez MJ, Planaguma M, Romani J, Curell R, de Moragas JM, et al. Clinical, histopathologic and therapeutic aspects of subepidermal autoimmune bullous diseases with IgG on the floor of salt-split skin. Int J Dermatol 2001;40:268-72.
[Google Scholar]
|
| 40. |
Jenkins RE, Rodenas J, Bhogal BS, Black MM. Optimal conditions of 1 M NaCl splitting technique to demonstrate basement membrane zone antigens in bullous pemphigoid, epidermolysis bullosa acquisita and linear IgA bullous dermatoses. Dermatology 1994;189 Suppl 1:133-4.
[Google Scholar]
|
| 41. |
Zenzo GD, Thoma-Vszynski S, Fontao L, Calabresi V, Hofmann SC, Hellmark T, et al. Multicentre prospective study of the humoral autoimmune response in bullous pemphigoid. Clin Immunol 2008;128:415-26.
[Google Scholar]
|
| 42. |
Hönigsmann H, Stingl G, Holubar K, Wolff K. Herpes getationis: Fine structural pattern of immunoglobulin deposits in the skin in vivo. J Invest Dermatol 1976;66:389-92.
[Google Scholar]
|
| 43. |
Gammon WR, Briggman RA. Epidermolysis bullosa acquisita and bullous systemic lupus eryhtematosus: Diseases of autoimmunity to type VII collagen. Dermatol Clin 1993;2:535- 47.
[Google Scholar]
|
| 44. |
Yaotia H, Briggman RA, Lawley TJ. Epidermolysis bullosa acquisita: ultrastructural and immunological studies. J Invest Dermatol 1981;76:285-92.
[Google Scholar]
|
| 45. |
Izuno GT. Cutaneous Immunofluorescence. Clin Lab Med 1986;6:85-102.
[Google Scholar]
|
| 46. |
Crowson AN, Magro C. The cutaneous pathology of lupus erythematosus: A review. J Cutan Pathol 2001;28:1-23.
[Google Scholar]
|
| 47. |
al-Suwaid AR, Venkataram MN, Bhushnurmath SR. Cutaneous lupus erythematosus: Comparison of direct immunofluorescence findings with histopathology. Int J Dermatol 1995;34:480-2.
[Google Scholar]
|
| 48. |
Sampaio MC, Oliveira ZN, Machado MC, Reis VM, Vilela MA. Discoid lupus erythematosus in children- a retrospective study of 34 patients. Pediatr Dermatol 2008;25:163-7.
[Google Scholar]
|
| 49. |
Rao Raghavendra, Balachandran C. Epidermal nuclear staining: A distinct reaction pattern in connective tissue diseases. Indian J Dermatol Venereol Leprol 2007;73;120-1.
[Google Scholar]
|
| 50. |
Velthuis PJ, Kater L, van der Tweel I, Meyling FG, Derksen RH, Hene RJ, et al. In vivo antinuclear antibody of the skin: Diagnostic significance and association with selective antinuclear antibodies. Ann Rheum Dis 1990;49:163-7.
[Google Scholar]
|
| 51. |
Arbesman J, Grover R, Helm TN, Beutner EH. Can direct immunofluorescence testing still be accurate if performed on biopsy specimens after brief inadvertent immersion in formalin? J Am Acad Dermatol 2011;65;106-1.
[Google Scholar]
|
| 52. |
Magro CM, Crowson AN, Regauer S. Mixed connective tissue disease: a clinical, histologic and immunofluorescence study of 8 cases. Am J Histopathol 1997;19:206-13.
[Google Scholar]
|
| 53. |
Shibeshi D, Blaszczyk M, Jarzabek-Chorzelska M, Jabloñska S, Chorzelski T. Immunopathologic findings in systemic sclerosis patients: Relation to clinical and immunologic relationships. Int J Dermatol 1989;28:650-6.
[Google Scholar]
|
| 54. |
Kumar L, Singh S, Goraya JS, Uppal B, Kakkar S, Walker R, et al. Henoch Schonlein purpura: The Chandigarh experience. Indian Pediatr 1998;35:19-25.
[Google Scholar]
|
| 55. |
Chhabra S, Minz RW, Rani L, Sharma N, Sakhuja V. Immune deposits in cutaneous lesions of Wegener's granulomatosis: predictor of an active disease. Indian J Dermatol 2011;56:758- 62.
[Google Scholar]
|
| 56. |
Kulthanan K, Jiamton S, Varothai S, Pinkaew S, Sutthipinittharm P. Direct immunofluorescence study in patients with lichen planus. Int J Dermatol 2007;46:1237-41.
[Google Scholar]
|
| 57. |
Yu-Hung Wu, Yang-Chih Lin. Cytoid bodies in cutaneous direct immunofluorescence examination. J Cutan Pathol 2007;34:481- 6.
[Google Scholar]
|
| 58. |
Ghularojanamontri L, Tuchinda P, Triwongwaranat D, Pinkaew S, Kulthanan K. Diagnostic significance of colloid body deposition in direct immunofluorescence. Indian J Dermatol Venereal Leprol 2010;76:373-7.
[Google Scholar]
|
| 59. |
Janjumratsang P, Phainupong D, Chanjanakijskul S, Roongphibulsopit P. Positive direct immunofluorescence and autoantibody profiles in psoriasis patients. J Dermatol 2008;35:508-13.
[Google Scholar]
|
Fulltext Views
57,220
PDF downloads
10,620





