Translate this page into:
Immunotherapy in viral warts with intradermal Bacillus Calmette–Guerin vaccine versus intradermal tuberculin purified protein derivative: A double-blind, randomized controlled trial comparing effectiveness and safety in a tertiary care center in Eastern India
2 Department of Pharmacology, Institute of Post Graduate Medical Education and Research, Kolkata, West Bengal, India
3 Department of Biochemistry, Medical College and Hospital, Kolkata, West Bengal, India
Corresponding Author:
Nilay Kanti Das
Department of Dermatology, Medical College and Hospital, 88, College Street, Kolkata - 700 073, West Bengal
India
drdasnilay@gmail.com
| How to cite this article: Podder I, Bhattacharya S, Mishra V, Sarkar TK, Chandra S, Sil A, Pal S, Kumar D, Saha A, Shome K, Bandyopadhyay D, Das NK. Immunotherapy in viral warts with intradermal Bacillus Calmette–Guerin vaccine versus intradermal tuberculin purified protein derivative: A double-blind, randomized controlled trial comparing effectiveness and safety in a tertiary care center in Eastern India. Indian J Dermatol Venereol Leprol 2017;83:411 |
Abstract
Background: Current therapeutic modalities for viral warts are mostly ablative and are limited by high recurrence rates besides being unsuitable for numerous lesions. Immunotherapy has the potential to overcome these limitations.Aims: The aim of this study was to compare the effectiveness and safety of Bacillus Calmette–Guerin vaccine versus tuberculin purified protein derivative in the immunotherapy of warts.
Methods: Patients received three doses of 0.1 ml of Bacillus Calmette–Guerin vaccine or tuberculin purified protein derivative intradermally over the deltoid region at 4-weekly intervals. They were followed-up for another month. Number of warts, complete cure rates and quality of life were assessed.
Results: A total of 60 patients were included. Complete clearance was noted in 16 (48.5%) out of 33 patients in the Bacillus Calmette–Guerin group and in 5 (18.5%) out of 27 in the tuberculin purified protein derivative group (P = 0.121). The number of lesions reduced statistically significantly from baseline in both the groups (P < 0.001) from the first follow-up visit onward (P < 0.05). The reduction was statistically significantly more in the Bacillus Calmette–Guerin group than in the tuberculin purified protein derivative group from the second follow-up onward. Dermatologic life quality index improved statistically significantly with both treatments. Adverse events (pain during injection, abscess formation and scarring at injection site) were more frequent with Bacillus Calmette–Guerin. No recurrence was seen after lesions cleared.
Limitations: Patients were not followed up for more than 4 weeks after treatment. We could not estimate the cytokine levels or the peripheral blood mononuclear cell proliferation in response to Bacillus Calmette–Guerin/tuberculin purified protein derivative injections.
Conclusion: Both intradermal Bacillus Calmette–Guerin and tuberculin purified protein derivative hold promise in the treatment of viral warts. Bacillus Calmette–Guerin may be more effective, though it had more adverse events in our study.
Introduction
Viral warts, caused by human papillomaviruses, are among the most common dermatological diseases and are notorious for being contagious, recurrent and recalcitrant. They can affect people of both sexes, no age group being spared. Treatment of warts becomes a challenge when they are numerous or present over inaccessible areas. There are many ablative modalities of therapy such as electrocautery, chemicocautery, cryotherapy, laser surgery, curettage and topical keratolytics. Most of these take months and many of them may result in pain, scarring, and recurrences.[1] Ablative therapies are also limited by the fact that they only remove visible lesions; non-visible infected tissues are not targeted, resulting in a high chance of recurrence.[2]
Recalcitrant, multiple viral warts often cause considerable concern to the patient and management of such cases is frustrating. In an attempt to deal with this challenge, dermatologists have come up with several intuitive management strategies, many of which may act by strengthening the immune system. Diphenylcyclopropenone, squaric acid dibutyl ester, imiquimod, tuberculin jelly, Candida antigen, autologous vaccines, human papillomavirus vaccination and measles, mumps and rubella vaccine have all been tried as immunotherapy. Diphenylcyclopropenone and squaric acid dibutyl ester have the potential to cause allergic contact dermatitis, urticarial lesions and pigmentary disturbances, while autologous vaccines have oncogenic potential.[1],[3],[4],[5],[6],[7]
Tuberculin antigen (purified protein derivative or tuberculin purified protein derivative, PPD) and Bacillus Calmette–Guerin (BCG) have garnered the attention of the dermatological fraternity for the treatment of multiple resistant warts. Purified protein derivative achieved 75% clearance of recalcitrant multiple viral warts and this was significantly better that the response in the saline control arm in a study by Abd-Elazeim et al. in Egypt.[8] Treatment of anogenital warts in pregnant women with intradermal purified protein derivative injection was found safe and effective with 47.5% demonstrating complete clearance and 37.5% partial response.[9]Bacillus Calmette–Guerin vaccination has been used for immunotherapy of viral warts in Iraq where a single-blind, placebo (distilled water) controlled study on 154 patients showed a significantly higher cure rate.[10]
Bacillus Calmette–Guerin was introduced as a prophylactic agent against tuberculosis, but is now also used in the treatment of malignant melanoma, transitional cell carcinoma of the bladder, alopecia areata and recurrent oral aphthosis.[10] It is thought to act by the stimulation of macrophages, T lymphocytes and natural killer cells. Toll-like receptor 7 may also play a role.
This study aimed to assess and compare the effectiveness of Bacillus Calmette–Guerin vaccination and tuberculin antigen (purified protein derivative or tuberculin purified protein derivative) for the treatment of multiple viral warts. We also aimed to look at the safety of these treatment regimens. We were unable to find any previous reports of a randomized trial of either with an active control.
Methods
The study was designed as a unicenter, double-blind, randomized, parallel group, active-controlled trial. Clearance from the Institutional Ethics Committee was obtained before the start of the study and written informed consent was obtained from all study participants or their legally authorized representatives. The study was registered in the Clinical Trial Registry-India (CTRI registration number: CTRI/2014/12/005244). The study was conducted from May 2014 for 8 consecutive months. All consecutive patients of either sex suffering from clinically diagnosed cutaneous warts and having more than five warts attending the dermatology outpatient department of Medical College and Hospital, Kolkata were included. Pregnant or lactating women, patients suffering from immunosuppression due to drug or disease, those with mucosal warts, nonconsenting patients, those with advanced diseases of vital organs, those unable to come for monthly follow-ups, and alcohol or other substance users were excluded from the study.
Visits
Screening visit
Patients were enrolled based on inclusion and exclusion criteria; written informed consent was obtained. All patients were referred to an integrated counseling and testing center and only those who were human immunodeficiency virus non-reactive were included. A thorough clinical examination was done. Routine hemogram, fasting blood glucose, serum urea, creatinine and liver function tests were also done.
Baseline visit
This visit was scheduled 7 days after the screening visit. The patients were randomized into two groups (Bacillus Calmette–Guerin or tuberculin purified protein derivative group) by a computer-generated random number table. The number of warts was counted and recorded in a standard case record form. Patients then received their first injection dose.
Follow-ups
Three follow-up visits were scheduled, at 4-weekly intervals. At each follow-up, the effectiveness parameters and adverse events were assessed. Bacillus Calmette–Guerin or tuberculin purified protein derivative injections were administered at the first and second follow-up visits. Injections were given till complete clearance of lesions, or till three sessions had been carried out.
Administration of Bacillus Calmette–Guerin/tuberculin purified protein derivative
According to the randomization, 0.1 ml of Bacillus Calmette–Guerin or tuberculin purified protein derivative was injected intradermally in the right arm (at the deltoid muscle insertion) with an insulin syringe.
Bacillus Calmette–Guerin
TUBERVAC ® (Manufacturer: Serum Institute of India Ltd., Pune, Maharashtra, India) was used. Each ml of reconstituted vaccine contains between 1 × 10[6] and 33 × 10[6] colony forming units. The freeze-dried, powdered Bacillus Calmette–Guerin vaccine was diluted with 1 ml of normal saline supplied with the vaccine vial. The vials were stored between 2° and 8° after reconstitution in a refrigerator and returned immediately after drawing the vaccine dose for each patient. Each prepared vial was used within 4 h of opening, as per standard norm.
Tuberculin purified protein derivative
APLISOL ® (Manufacturer: JHP Pharmaceuticals, Rochester, USA) was used. Aplisol is bioequivalent in potency to the standard purified protein derivative-S* (5 tuberculin units/0.1 ml) of the U.S. Public Health Service, National Centers for Disease Control. The vial was stored between 2° and 8° in a refrigerator and returned immediately after drawing each patient's dose.
Effectiveness parameters
The primary effectiveness parameter was the number of visible warts on the skin. The secondary effectiveness parameters were the number of patients showing complete resolution of warts and the quality of life assessed by the vernacular version of the Dermatology Life Quality Index (with permission from the developer Prof. Andrew Finlay).
Safety parameters
Vital signs and adverse events reported by the patient or elicited by the clinician were assessed at each follow-up. Laboratory parameters were recorded at baseline and third follow-up.
Randomization and allocation concealment
Simple unstratified randomization to divide the patients into two groups was done using a computer-generated random number table. Concealment of randomization allocation was done by the sequentially numbered opaque sealed envelopes technique.
Blinding
After evaluation, the investigator referred the patient to an independent coordinator seated in another room. The coordinator was responsible for randomization, filling the insulin syringe with the appropriate trial medication and dispensing it to the investigator. The treating physician injected the medicine and noted clinical parameters. Both the investigator and the patients were blind to treatment allocation.
Sample size estimation
There were 27 patients in each treatment group. Sample size was calculated considering complete clearance of warts in 39.7% with Bacillus Calmette–Guerin and 75% with tuberculin purified protein derivative, with 80% power and 0.05 probability of type 1 error.[8],[10] Considering a 10% possible dropout rate, this translated into a recruitment target of approximately thirty patients per group, or sixty patients overall.
Statistical analysis
Continuous variables (age, duration of illness) were compared between the groups by the independent samples t-test and within each group by a paired t-test. Mann–Whitney U-test and Wilcoxon matched-pairs signed-ranks test were employed for comparisons of unpaired and paired nonparametric data (number of warts, presence of Bacillus Calmette–Guerin immunization scar). Friedman's analysis of variance was carried out with non-parametric data for within-group repeated measures comparisons, followed by a post hoc Dunn's test. Categorical data were compared between the groups by Chi-square test or Fisher's exact test as appropriate. MedCalc version 11.6 (Mariakerke, Belgium: MedCalc Software, 2011) and GraphPad Prism version 5 (San Diego, California: GraphPad Software Inc., 20057) software were used for statistical analysis. P< 0.05 was considered statistically significant.
Effectiveness analysis was done on a modified intention-to-treat basis with subjects reporting for at least one post-baseline follow-up visit. Missing values were dealt with by the last observation carried forward strategy. Pre- and post-treatment laboratory values were compared in patients for whom both sets of data were available. For other safety analysis, all patients who had received at least one dose of a study drug (essentially all sixty patients) were considered.
Results
The participant flow is depicted in [Figure - 1]. Sixty patients were randomized into two groups. Five patients were lost to follow-up. Of them, two patients in the Bacillus Calmette–Guerin group complained of scarring and did not come for subsequent follow-ups. In the tuberculin purified protein derivative group, one patient said that pain was the reason for his absence from follow-ups, one had migrated to a neighboring state on account of a family matter, and the third could not be contacted over the phone.
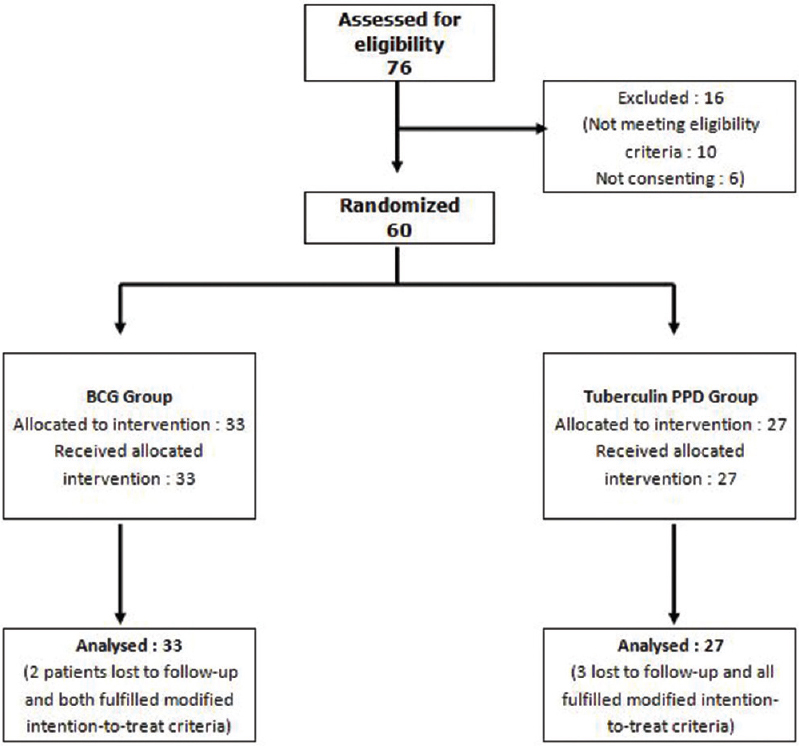 |
| Figure 1: Flow of study participants |
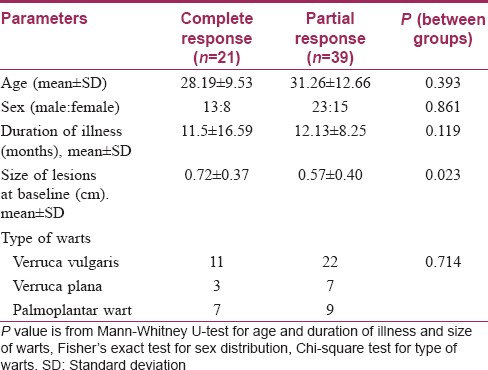
Men outnumbered women and patients were mostly in their late twenties or thirties. Both groups were comparable with respect to age, sex, residence (rural or urban) and income (above poverty line, below poverty line). The duration of illness was 11 ± 13.64 months in the Bacillus Calmette–Guerin group and 13.15 ± 8.85 months in the tuberculin purified protein derivative group with no significant difference between them. Both groups were also comparable in terms of the mean size of lesions at baseline (P = 0.132) and the type of warts seen (P = 0.116) [Table - 1].
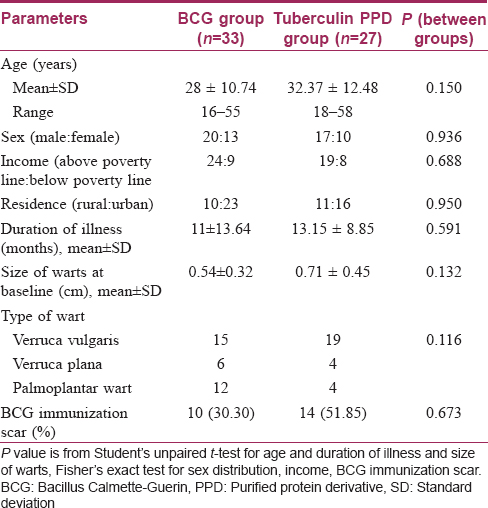
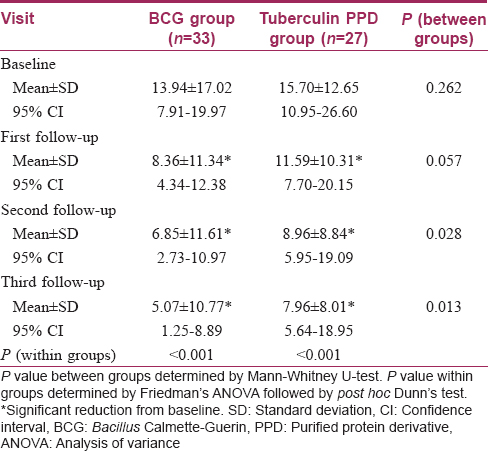
The number of warts was comparable initially in the two treatment arms. In the Bacillus Calmette–Guerin group, the number of warts significantly decreased from the first follow-up onwards (P < 0.001) till the end of the study [Figure 2a], [Figure 2b] and [Figure 3a], [Figure 3b]. Similar results were obtained in the tuberculin purified protein derivative group. However, when the groups were compared, the reduction in the mean number of warts was found to be significantly more in the Bacillus Calmette–Guerin group from the second follow-up onwards [Table - 2].
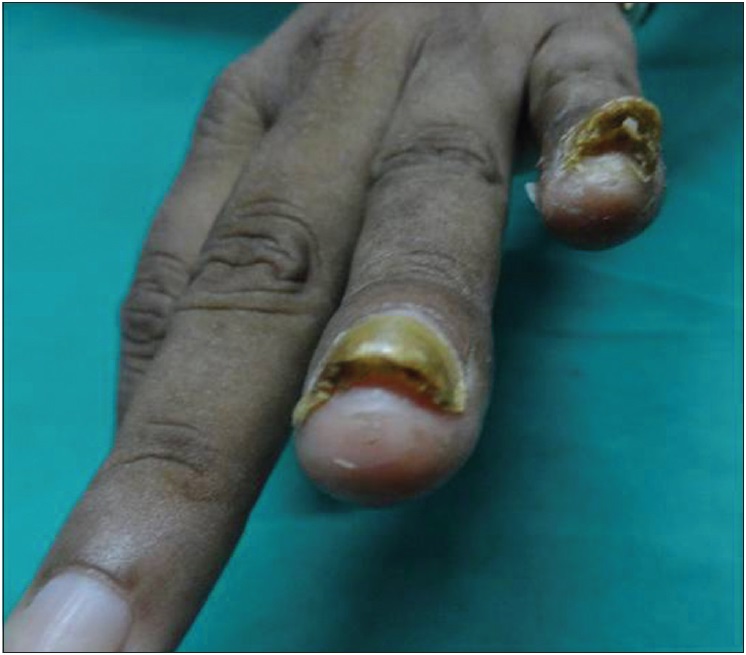 |
| Figure 2a: Subungual warts: pretreatment |
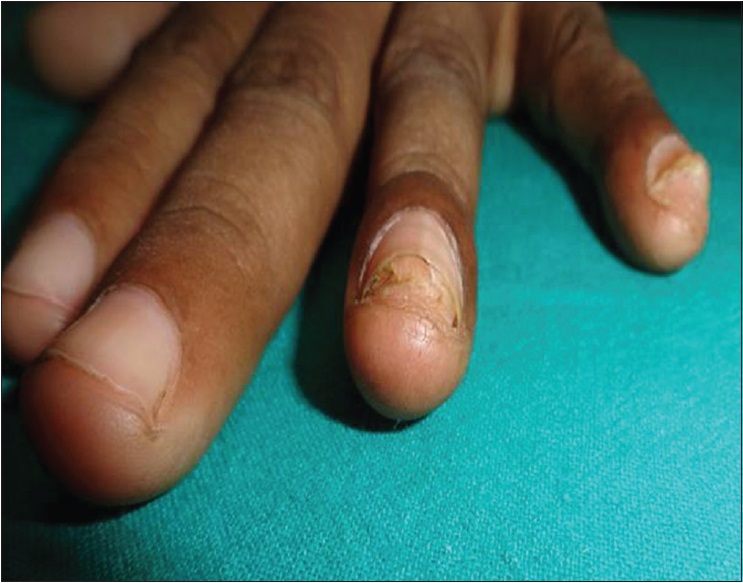 |
| Figure 2b: Subungual warts: complete clearance of difficult-to-treat warts with three doses of Bacillus Calmette Guerin (at 12 weeks of treatment) |
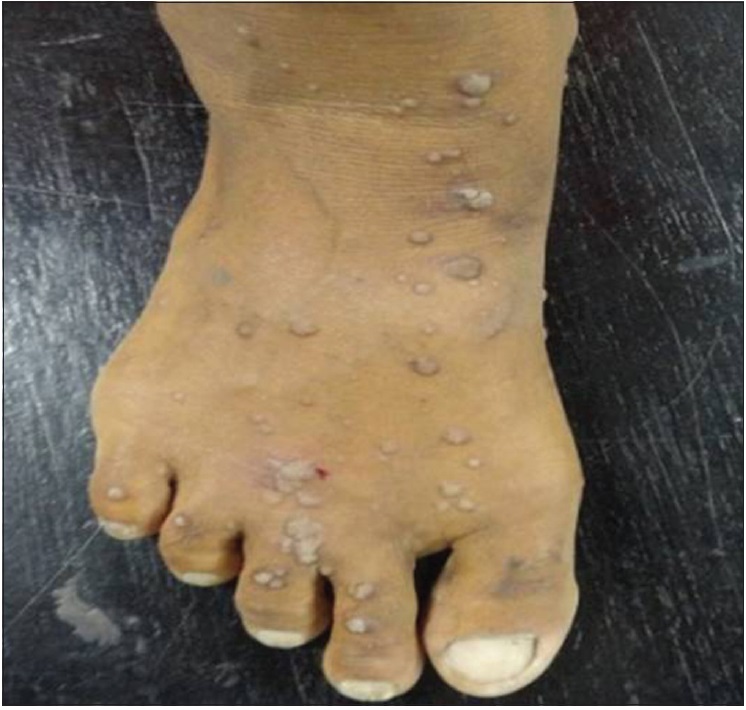 |
| Figure 3a: Pretreatment photograph showing multiple warts (verucca plana) on foot |
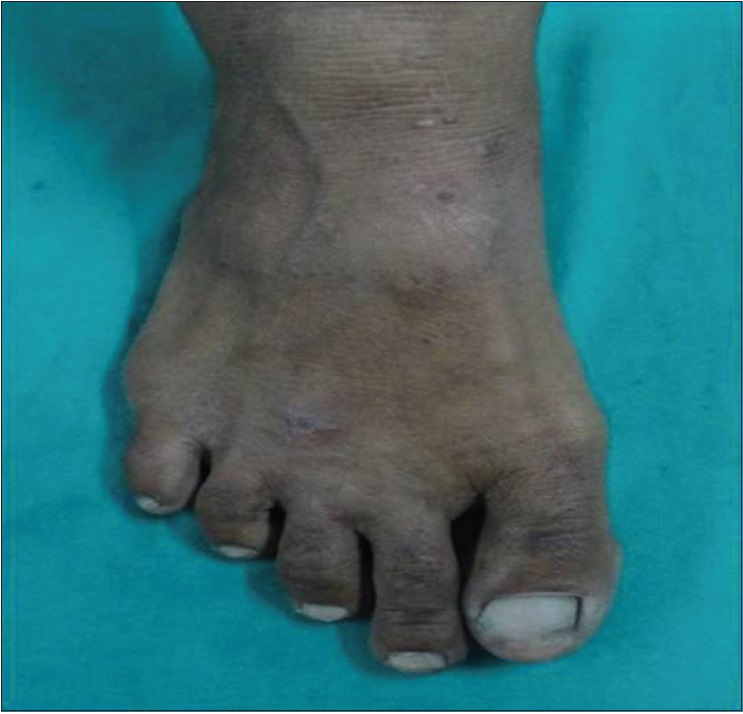 |
| Figure 3b: Posttreatment photograph showing complete clearance of multiple warts (verucca plana) on foot with three doses of tuberculin purified protein derivative (at 12 weeks of treatment) |
Complete resolution of warts could be seen from the first follow-up onward in the Bacillus Calmette–Guerin group; at the end of the study, 48.5% of patients achieved complete remission in this group compared to 18.5% in the tuberculin purified protein derivative group (P = 0.028) [Table - 3]. Among the sixty patients observed, complete resolution of warts was obtained in 21 patients while the remaining 39 responded partially. Age, sex, duration and type of warts were all comparable amongst the complete cure and partial response categories. Interestingly, complete responders had had significantly larger warts than had those who responded partially [Table - 4]. No recurrences were seen in either of the two groups.
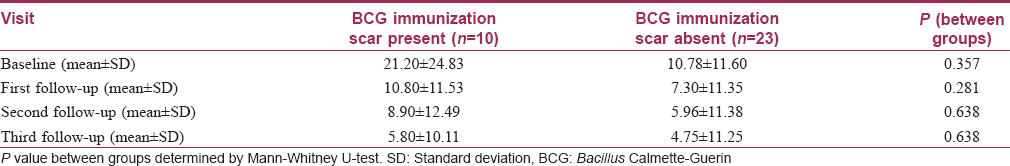
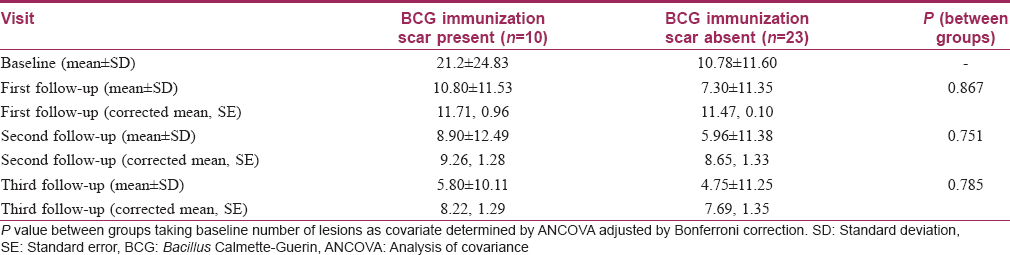
Bacillus Calmette–Guerin immunization scar was present in 30.3% of patients in the Bacillus Calmette–Guerin group and in 51.9% in the tuberculin purified protein derivative group [Table - 1]. Subgroup analysis with the presence of a Bacillus Calmette–Guerin immunization scar as the grouping variable showed no significant differences in the reduction of the number of warts between the two subgroups at all follow-ups in the Bacillus Calmette–Guerin group [Table - 5]. A similar subgroup analysis in the tuberculin purified protein derivative group showed a significant difference of the number of warts at baseline itself (P = 0.029); analysis of covariance with the baseline as covariate yielded no significant differences between these subgroups [Table - 6].
The quality of life (assessed by the Dermatology Life Quality Index) was comparable (P = 0.667) in both the treatment arms at baseline. The index had improved significantly from baseline at the end of the study with both Bacillus Calmette–Guerin (P = 0.004) and purified protein derivative (P = 0.005), but an intergroup comparison showed no significant difference between the two treatment groups (P = 0.482).
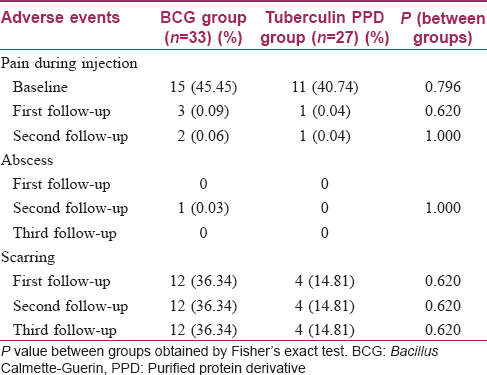
Adverse events were observed more frequently in the Bacillus Calmette–Guerin group. More patients complained of pain during injection in this group, though this was not statistically significant (P = 0.796). An abscess occurred at the injection site in one patient in the Bacillus Calmette–Guerin group. Scar formation was also found to be higher in the Bacillus Calmette–Guerin group (nearly 36%), but this too was not statistically significant (P = 0.620) [Table - 7]. One patient in the Bacillus Calmette–Guerin group developed two scars during therapy. Laboratory parameters were within normal limits and comparable between the groups. None of the patients experienced any serious adverse event during the period of the trial.
Discussion
Immunotherapy for warts employs the ability of the immune system to recognize certain viral, bacterial and fungal antigens that induce a delayed-type hypersensitivity reaction in a previously sensitized individual, not only to the antigens themselves but also against the wart virus, which increases the ability of the immune system to recognize and clear the human papillomavirus.[2],[11] Injection of the antigen results in peripheral blood mononuclear cell proliferation, promoting Th1 cytokine responses, particularly interferon-gamma and interlekuin-2. This results in activation of cytotoxic T cells and natural killer cells that help to eradicate human papillomavirus-infected cells.[12] It is also proposed that antigen immunotherapy can stimulate tumor necrosis factor-α and interlekuin-1 release, downregulating gene transcription of human papillomavirus.[13] The ability of the antigen to change the cytokine milieu to a Th1 response pattern triggering a cell-mediated immune response against the human papillomavirus seems to be the cornerstone of immunotherapy.
Immunotherapy addresses the limitations of ablative therapy in that it enhances the cell-mediated immune response that clears the virus-infected tissue irrespective of whether it is visible or not. It might also be able to target warts situated away from the site of the immunotherapeutic injection and therefore help in treating multiple warts, warts on inaccessible sites or sites where ablative therapy is difficult (e.g., subungual or periungual regions).
Agents used for intradermal/intralesional immunotherapy include extracted proteins (e.g., tuberculin), bacterial agents (e.g., Bacillus Calmette–Guerin, Mycobacterium w vaccine), fungal agents (e.g., Candida albicans, Trichophyton), viral agents (measles, mumps and rubella, and autoinoculation of warty tissue).[7],[8],[9] Some investigators favor determining the sensitization status of the individual with a presensitization test while others feel that this approach is not practical in view of patient compliance and increased costs.[14] India being a country where the prevalence of tuberculosis is very high (249/100,000 population) with an estimated 40% of the population infected with M. tuberculosis and where routine immunization against tuberculosis is in practice, it can be argued that the Indian population is widely sensitized to M. tuberculosis.[15] We therefore chose a related antigen (Bacillus Calmette–Guerin or tuberculin purified protein derivative), eliminating the need of a sensitization test.
Both forms of immunotherapy appeared effective in our study, with significant responses seen 4 weeks onward. Our findings also indicate that both can be advocated irrespective of the patients' Bacillus Calmette–Guerin immunization status. However we did find that Bacillus Calmette–Guerin was more effective than tuberculin purified protein derivative in terms of complete clearance and reduction in numbers of warts, perhaps because of greater numbers of cross-reacting epitopes being present on the whole bacterial antigen of M. bovis (in Bacillus Calmette–Guerin) than in the protein extract of M. tuberculosis (in tuberculin purified protein derivative).
Reduction in wart numbers continued even after the three dosages were complete (as evident by the follow-up visit 4 weeks after the last injection). Complete cure was obtained in patients with larger warts, while the duration or type of warts did not appear to make a difference in our study. It may be hypothesized that larger warts have larger viral loads and hence greater epitope sharing with the cross-reacting antigen. However some previous studies had different results, with larger warts, those present for longer durations and plantar warts showing less favorable outcomes.[16],[17]
Scarring, which occurred in many of our cases, can cause discontent among patients; they should be made aware of this possible adverse effect before beginning therapy. Unexposed parts of the body (e.g., thighs) can be chosen for injections in those who are concerned about scarring. The study was limited by the fact that patients could not be followed up for more than 4 weeks. We also could not estimate cytokine levels or peripheral blood mononuclear cell responses to Bacillus Calmette–Guerin/tuberculin purified protein derivative injections due to infrastructural constraints. It needs to be mentioned that one vial of Bacillus Calmette–Guerin when reconstituted can be used for ten patients and has to be utilized within 4 h. It is therefore advisable to club patients for Bacillus Calmette–Guerin immunotherapy on one particular day to prevent wastage. This limitation is absent with tuberculin purified protein derivative.
Conclusion
Both Bacillus Calmette–Guerin and tuberculin purified protein derivative given intradermally at 4-weekly intervals show positive responses and are well-tolerated therapeutic options for viral warts. Bacillus Calmette–Guerin was found to be more effective than tuberculin purified protein derivative though it has the limitations of causing more pain and scarring. Response starts occurring 4 weeks after the first injection and continues even after completion of the three-dose schedule. Since the injections are given at a site away from the lesions being treated, this modality is suited for multiple lesions and for lesions in inaccessible and difficult-to-treat sites, such as the subungual or periungual regions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. | Signore RJ. Candida albicans intralesional injection immunotherapy of warts. Cutis 2002;70:185-92. [Google Scholar] |
| 2. | Nofal A, Salah E, Nofal E, Yosef A. Intralesional antigen immunotherapy for the treatment of warts: Current concepts and future prospects. Am J Clin Dermatol 2013;14:253-60. [Google Scholar] |
| 3. | Baandrup L, Blomberg M, Dehlendorff C, Sand C, Andersen KK, Kjaer SK. Significant decrease in the incidence of genital warts in young Danish women after implementation of a national human papillomavirus vaccination program. Sex Transm Dis 2013;40:130-5. [Google Scholar] |
| 4. | Mikolajczyk RT, Kraut AA, Horn J, Schulze-Rath R, Garbe E. Changes in incidence of anogenital warts diagnoses after the introduction of human papillomavirus vaccination in Germany-an ecologic study. Sex Transm Dis 2013;40:28-31. [Google Scholar] |
| 5. | Canfell K, Chesson H, Kulasingam SL, Berkhof J, Diaz M, Kim JJ. Modeling preventative strategies against human papillomavirus-related disease in developed countries. Vaccine 2012;30 Suppl 5:F157-67. [Google Scholar] |
| 6. | Gamil H, Elgharib I, Nofal A, Abd-Elaziz T. Intralesional immunotherapy of plantar warts: Report of a new antigen combination. J Am Acad Dermatol 2010;63:40-3. [Google Scholar] |
| 7. | Nofal A, Nofal E. Intralesional immunotherapy of common warts: Successful treatment with mumps, measles and rubella vaccine. J Eur Acad Dermatol Venereol 2010;24:1166-70. [Google Scholar] |
| 8. | Abd-Elazeim FM, Mohammed GF, Fathy A, Mohamed RW. Evaluation of IL-12 serum level in patients with recalcitrant multiple common warts, treated by intralesional tuberculin antigen. J Dermatolog Treat 2014;25:264-7. [Google Scholar] |
| 9. | Eassa BI, Abou-Bakr AA, El-Khalawany MA. Intradermal injection of PPD as a novel approach of immunotherapy in anogenital warts in pregnant women. Dermatol Ther 2011;24:137-43. [Google Scholar] |
| 10. | Sharquie KE, Al-Rawi JR, Al-Nuaimy AA, Radhy SH. Bacille Calmette-Guerin immunotherapy of viral warts. Saudi Med J 2008;29:589-93. [Google Scholar] |
| 11. | Bacelieri R, Johnson SM. Cutaneous warts: An evidence-based approach to therapy. Am Fam Physician 2005;72:647-52. [Google Scholar] |
| 12. | Horn TD, Johnson SM, Helm RM, Roberson PK. Intralesional immunotherapy of warts with mumps, Candida, and Trichophyton skin test antigens: A single-blinded, randomized, and controlled trial. Arch Dermatol 2005;141:589-94. [Google Scholar] |
| 13. | Böhle A, Büttner H, Jocham D. Primary treatment of condylomata acuminata with viable bacillus Calmette-Guerin. J Urol 2001;165:834-6. [Google Scholar] |
| 14. | Johnson SM, Roberson PK, Horn TD. Intralesional injection of mumps or Candida skin test antigens: A novel immunotherapy for warts. Arch Dermatol 2001;137:451-5. [Google Scholar] |
| 15. | TB India 2012. Revised National TB Control Program, Annual Status Report. Central TB Division. Directorate General of Health Services Ministry of Health and Family Welfare, New Delhi, India; March, 2012. [Google Scholar] |
| 16. | Mulhem E, Pinelis S. Treatment of nongenital cutaneous warts. Am Fam Physician 2011;84:288-93. [Google Scholar] |
| 17. | Clifton MM, Johnson SM, Roberson PK, Kincannon J, Horn TD. Immunotherapy for recalcitrant warts in children using intralesional mumps or Candida antigens. Pediatr Dermatol 2003;20:268-71. [Google Scholar] |
Fulltext Views
6,070
PDF downloads
2,236





