Translate this page into:
Immunotherapy using purified protein derivative in the treatment of warts: An open uncontrolled trial
2 Dr. Saoji's Skin Clinic, Nagpur, Maharashtra, India
Correspondence Address:
Vikrant Saoji
22, Sukhad, Dandige Layout, Shankar Nagar, Nagpur - 440 010, Maharashtra
India
| How to cite this article: Saoji V, Lade NR, Gadegone R, Bhat A. Immunotherapy using purified protein derivative in the treatment of warts: An open uncontrolled trial. Indian J Dermatol Venereol Leprol 2016;82:42-46 |
Abstract
Background: Warts are known to clear spontaneously with the development of cell-mediated immunity (CMI) to the virus. Purified protein derivative (PPD) of tuberculin bacilli has been used as a non-specific stimulant of CMI to achieve this outcome. Aim: To study the effect of PPD in the treatment of warts. Methods: Patients with difficult-to-treat warts were selected for immunotherapy. Each patient received 2.5 TU of PPD intralesionally in a few warts. A total of four sessions were given at 2 weekly intervals and patients were followed up for 6 months after the last dose. Results: Sixty-one patients were recruited of which 55 completed 6 months follow up and were available for analysis. Of these, 25 had verruca vulgaris, 18 had verruca plana and 12 had plantar warts. Forty two (76%) patients showed complete clearance after four sessions while the remaining 13 (24%) patients were non-responders. One patient developed a recurrence after total clearance during the follow-up period. Adverse effects were erythema, edema and pain at the site of injections. Limitations: As this was an uncontrolled trial, there is no comparison with a non-intervention group. Also, a Mantoux test was not done due to practical difficulties. Conclusion: Immunotherapy with PPD is helpful in the treatment of cutaneous warts.Introduction
Warts are caused by human papillomaviruses (HPVs) which infect keratinocytes. Local destruction of the warts is a commonly employed treatment modality which may be performed by chemical or electric cauterization, carbon dioxide laser or cryotherapy. All these modalities have a potential to cause dyspigmentation and/or scarring and may be associated with frequent recurrences. They also have limited use in multiple warts, facial warts and palmo-plantar warts.[1] Spontaneous regression is known to occur in warts due to the development of cell-mediated immunity (CMI) to the virus and such lesions demonstrate prominent lymphocytic infiltration in the dermis.[1],[2] An immune response is essential for clearance of warts.[1],[3],[4] Immunotherapy is an innovative approach to treat warts which relies on the principle of enhancement of cell-mediated immunity. It has been carried out using various antigens like mumps, Candidin and Trichophytin.[5],[6],[7] The objective of this study was to evaluate the effect of purified protein derivative (PPD) of Mycobacterium tuberculosis as immunotherapy in difficult-to-treat warts.
Methods
The study was done at the author's clinic after obtaining ethical clearance from an independent ethical committee. Patients with difficult-to-treat warts such as palmoplantar warts, periungual warts, facial warts (>10 lesions), verucca vulgaris (>10 lesions) and verruca plana (>10 lesions) who were untreated or were off treatment for at least 1 month were recruited prospectively between July 2010 and March 2012. Those who declined to participate, pregnant women, patients on immunosuppressive drugs and patients with active systemic illness or infection were excluded. History of tuberculosis or past systemic illnesses were not considered as exclusion criteria.
After informed consent from the patient (or parent if the patient was a minor), 2.5 TU of PPD was injected into each lesion. In case of multiple lesions, a maximum of 10 representative lesions covering all the sites and a maximum of 25 TU of PPD was injected during each session. A total of four sessions were planned at an interval of 2 weeks irrespective of whether they had had a complete response. Patients reporting late for a session were continued on the regimen provided they were late by less than 7 days. Every patient was asked to complete the schedule of four sessions even if their lesions cleared earlier. Those patients who had complete resolution before four sessions were given 2.5 TU of PPD on the site of cleared lesions. Clinical response was assessed and photographs were taken during each visit. Patients were followed up at 1 month after the last dose. After 6 months, all the patients were called telephonically to enquire about any recurrence and 26 patients who returned for follow up were examined. Mantoux test was not done because of practical difficulties as many patients were staying far away and it was not possible for them to report after 48 h for the reading. No other systemic or topical anti-wart medications were allowed to be used simultaneously. The response to treatment was evaluated by observing all the warts on injected and non-injected sites. The response was graded as:
- Responder: Total clearance of the lesions, and
- Non-responder: No or partial clearance of the lesions.
Results
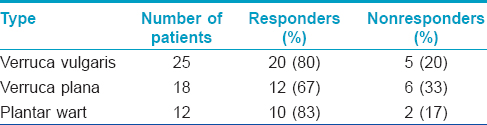
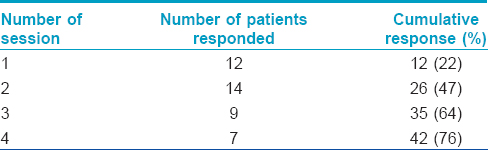
Seventy nine patients were screened of whom 18 patients were excluded as they were not willing to follow the study protocol; the remaining 61 patients were enrolled in the study. Six patients did not come for the second visit and were considered as dropouts. A total of 55 patients were available for analysis which included 40 males and 15 females aged 4–57 years with a mean age of 28.3 years. Twenty five patients had verruca vulgaris, 18 patients had verruca plana on face and 12 patients had plantar warts. The number of lesions ranged from 2 to >100. All the patients with verruca plana and verruca vulgaris had multiple lesions (>10). Forty two (76%) patients showed complete clearance after four sessions [[Figure - 1]a and [Figure - 1]b,[Figure - 2]a and [Figure - 2]b, [Figure - 3]a and [Figure - 3]b. while 13 (24%) patients were non-responders. The response rate of various types of warts are shown in [Table - 1]. Number of injections and response rate is shown in [Table - 2].
 |
| Figure 1: Verruca plana (a) Before treatment and (b) Total clearance after second injection |
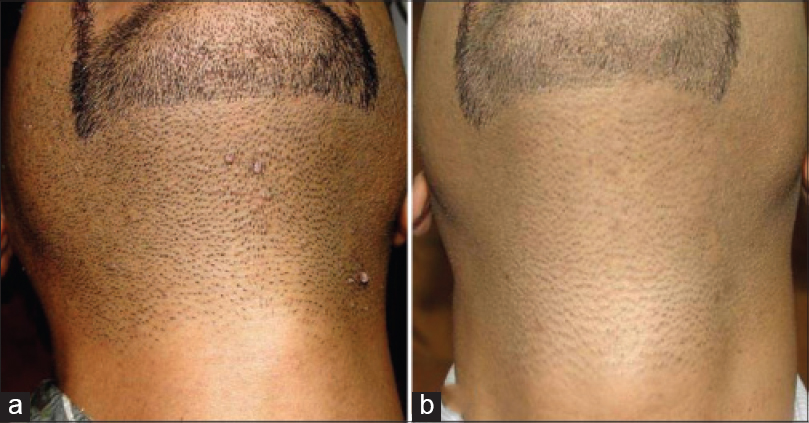 |
| Figure 2: Verruca vulgaris (a) Before treatment and (b) Total clearance after third injection |
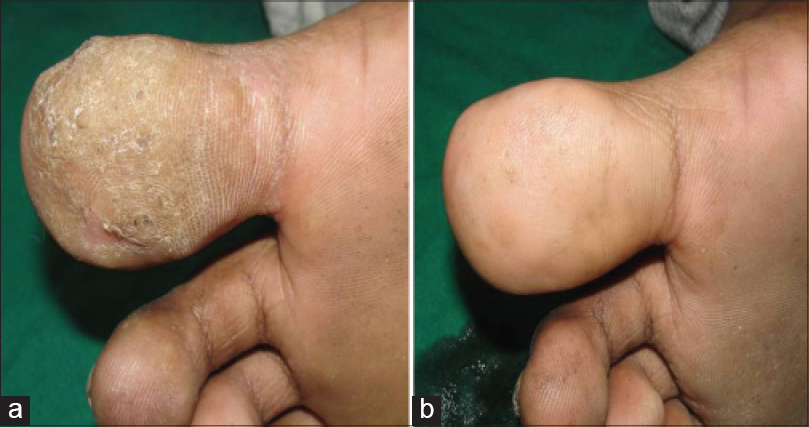 |
| Figure 3: Plantar wart (a) Before treatment and (b) Total clearance after third injection |
Purified protein derivative injection was well tolerated. The most commonly observed side effects were mild redness and swelling at the injection site seen in 13 patients which lasted for 4–7 days. After injection of an eyebrow lesion, one female patient developed lid edema that responded to cold compresses [Figure - 4]. Constitutional symptoms including low grade fever and body ache developed in one patient who had received 25 TU PPD and required analgesics for 2 days. One child developed an eczematous lesion at the site of injection which required topical fluticasone cream [Figure5]. No scarring or pigmentary change was observed in any patient. One patient of verucca vulgaris developed recurrence during the follow-up period.
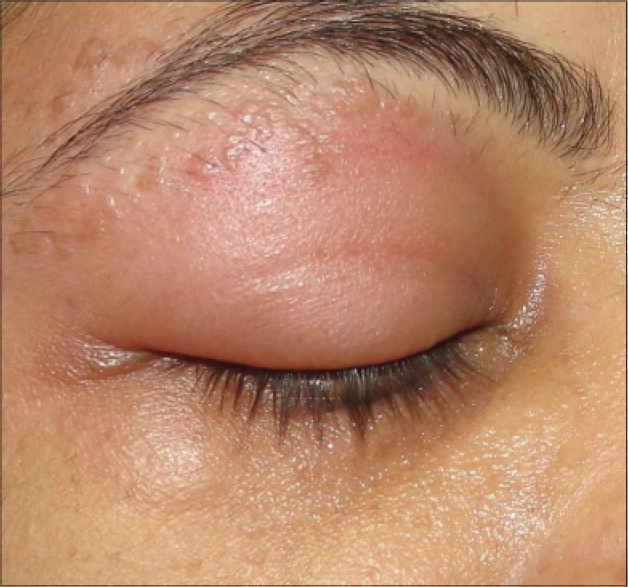 |
| Figure 4: Lid edema developing at the site of injection |
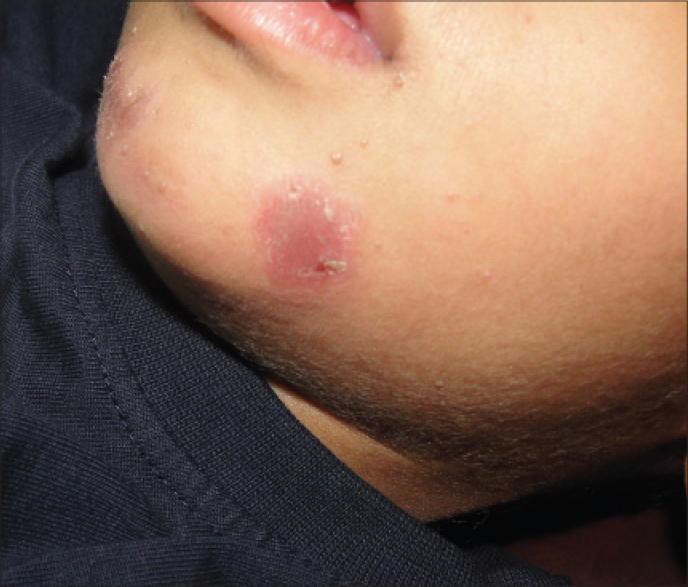 |
| Figure 5: Eczematous reaction at the site of injection |
Discussion
Local tissue destruction is a commonly employed method in the treatment of warts. However, it is not practical for multiple lesions, palmo-plantar and facial lesions because of associated scarring or pigmentation.[1] None of the destructive methods available are precise enough to destroy only the epidermis and hence scarring is almost inevitable with the use of these modalities. With immunotherapy, warts have been found to regress without any scarring and hence it is considered useful for plantar, facial and genital lesions.[7],[8],[9] In addition, the recurrence rate following immunotherapy is minimal as compared to destructive therapies.[1],[8],[9],[10]
Cell-mediated immunity plays a protective role against viral, fungal and mycobacterial infection. Warts are known to clear spontaneously and a Cochrane review found a cure rate of 22% in the placebo arm.[1],[4] Hence, to stimulate cell mediated immunity viral, fungal or mycobacterial antigens and vaccines have been used.[5], 7, [9],[10],[11],[12] Because of the high prevalence of tuberculosis infection in developing countries like India, it is easy to induce a positive cell mediated immunity response with PPD, which was the reason for selecting PPD for immunostimulation in our study. Injection of PPD stimulates cell mediated immunity non-specifically through activation of Th1 cytokines, natural killer cells and cytotoxic T cells and is found to be effective against all types of warts such as verruca plana, verruca vulgaris and plantar warts irrespective of the serotype of HPV.[8],[9],[13] It was observed that immunotherapy injections lead to significantly greater clearance of warts than normal saline injectionsindicating that it is the specific effect of cell mediated immunity stimulation and not the effect of injection alone.[11],[12] In a study of 233 patients, the response rate to immunotherapy was found to be unrelated to age and gender of the patient, type of warts and HLA typing.[12] It is believed that injection of PPD not only stimulates the local immunity but also leads to circulation of activated T cells in the body leading to clearance of injected as well as non-injected, distant warts.[8],[9],[13]
Purified protein derivative is a protein derivative and does not contain any viable organisms hence it can be used safely in children and pregnant women.[8],[13] Eassa et al., reported the use of PPD for the treatment of anogenital warts in pregnancy with a high success rate and without any major side effects confirming its efficacy and safety in pregnancy.[8] Though the product insert warns against use in pregnancy, no teratogenic effects of Mantoux testing during pregnancy have been documented.[14] The risk of hypersensitivity is very rare occurring in less than 1/million doses.[15] The maximum reported safe dose of PPD is 88 TU. No linear correlation has been observed between tuberculin dose and skin reaction.[16] The largest dose used in our study was 25 TU in one patient who experienced constitutional symptoms which were easily managed with anti-inflammatory drugs. There are variations in various studies regarding the dose, the interval between sessions and the number of sessions [Table - 3] and further studies are needed to develop a standard protocol. We decided to use an interval of 2 weeks as the usual time taken for local induration to resolve after the Mantoux test is 5–10 days.
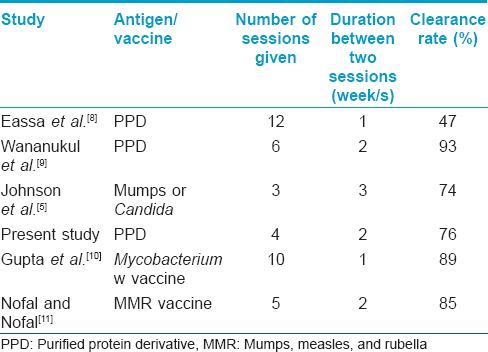
[Table - 3] compares the response rate of various antigens/vaccines used in previous studies with the present study. In our study 42 (76%) patients had complete clearance of warts in only 4 sessions. The higher and quicker response rate may be due to the use of intralesional injections in multiple lesions and the higher quantity of PPD injected which was not done in earlier studies. The present study and other studies reveal that intralesional injections given on multiple lesions and over a number of sittings gives better and faster results.[8],[9],[10],[11],[12] Topical immunotherapy with tuberculin jelly had shown a lower response rate of 57% hence it is better to use the intralesional route of administration for higher clearance.[17]
Purified protein derivative immunotherapy was well tolerated by our patients. In our study, only 1 (1.8%) patient of verruca vulgaris developed recurrence during the follow-up period similar to that observed in another study.[9] The limitations of our study include absence of a control group and Mantoux test not having been done and hence we could not correlate the response rate with Mantoux positivity.
This open, uncontrolled trial suggests that intralesional immunotherapy with tuberculin PPD may be a useful treatment of cutaneous warts. It is widely available, cheap and easy to use.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Sterling JC, Handfield-Jones S, Hudson PM, British Association of Dermatologists. Guidelines for the management of cutaneous warts. Br J Dermatol 2001;144:4-11.
[Google Scholar]
|
| 2. |
Xu X, Erickson L, Chen L, Elder D. Diseases caused by virus's. In: Elder D, Elenitsas R, Johnson B Jr, Murphy G, Xu X, editors. Lever's Histopathology of the Skin. 10th ed. Philadelphia: Lippincott William and Wilkins; 2009. p. 653.
[Google Scholar]
|
| 3. |
Bacelieri R, Johnson SM. Cutaneous warts: An evidence-based approach to therapy. Am Fam Physician 2005;72:647-52.
[Google Scholar]
|
| 4. |
Gibbs S, Harvey I, Sterling JC, Stark R. Local treatments for cutaneous warts. Cochrane Database Syst Rev 2006;3:CD001781.
[Google Scholar]
|
| 5. |
Johnson SM, Roberson PK, Horn TD. Intralesional injection of mumps or Candida skin test antigens: A novel immunotherapy for warts. Arch Dermatol 2001;137:451-5.
[Google Scholar]
|
| 6. |
Johnson SM, Horn TD. Intralesional immunotherapy for warts using a combination of skin test antigens: A safe and effective therapy. J Drugs Dermatol 2004;3:263-5.
[Google Scholar]
|
| 7. |
Clifton MM, Johnson SM, Roberson PK, Kincannon J, Horn TD. Immunotherapy for recalcitrant warts in children using intralesional mumps or Candida antigens. Pediatr Dermatol 2003;20:268-71.
[Google Scholar]
|
| 8. |
Eassa BI, Abou-Bakr AA, El-Khalawany MA. Intradermal injection of PPD as a novel approach of immunotherapy in anogenital warts in pregnant women. Dermatol Ther 2011;24:137-43.
[Google Scholar]
|
| 9. |
Wananukul S, Chatproedprai S, Kittiratsacha P. Intralesional immunotherapy using tuberculin PPD in the treatment of palmoplantar and periungual warts. Asian Biomed 2009;3:739-43.
[Google Scholar]
|
| 10. |
Gupta S, Malhotra AK, Verma KK, Sharma VK. Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts: An open label pilot study. J Eur Acad Dermatol Venereol 2008;22:1089-93.
[Google Scholar]
|
| 11. |
Nofal A, Nofal E. Intralesional immunotherapy of common warts: Successful treatment with mumps, measles and rubella vaccine. J Eur Acad Dermatol Venereol 2010;24:1166-70.
[Google Scholar]
|
| 12. |
Horn TD, Johnson SM, Helm RM, Roberson PK. Intralesional immunotherapy of warts with mumps, Candida, and Trichophyton skin test antigens: A single-blinded, randomized, and controlled trial. Arch Dermatol 2005;141:589-94.
[Google Scholar]
|
| 13. |
Elela IM, Elshahid AR, Mosbeh AS. Intradermal vs intralesional purified protein derivatives in treatment of warts. Golf J Deramatol Venereol 2011;18:21-6.
[Google Scholar]
|
| 14. |
Screening for tuberculosis and tuberculosis infection in high-risk populations. Recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Morb Mortal Wkly Rep 1995;44:19-34.
[Google Scholar]
|
| 15. |
Froeschle JE, Ruben FL, Bloh AM. Immediate hypersensitivity reactions after use of tuberculin skin testing. Clin Infect Dis 2002;34:E12-3.
[Google Scholar]
|
| 16. |
Dimoliatis ID, Liaskos CA. Six Mantoux tuberculin skin tests with 1, 2, 5, 10, 20, and 50 units in a healthy male without side-effects-Is skin reaction a linear function of tuberculin dose? Cases J 2008;1:115.
[Google Scholar]
|
| 17. |
Lahti A, Hannuksela M. Topical immunotherapy with tuberculin jelly for common warts. Arch Dermatol Res 1982;273:153-4.
[Google Scholar]
|
Fulltext Views
14,751
PDF downloads
3,876





