Translate this page into:
In vivo antinuclear antibodies of the skin
Correspondence Address:
Seema Chhabra
Room no. 29, 4th floor, Research Block-A, PGIMER, Sector-12, Chandigarh
India
| How to cite this article: Arora SK, Chhabra S. In vivo antinuclear antibodies of the skin. Indian J Dermatol Venereol Leprol 2012;78:116-118 |
Introduction
Direct immunofluorescence (DIF) testing occupies an important place in the diagnosis and evaluation of many diseases. It is most commonly employed on skin biopsies to diagnose the autoimmune bullous disorders of the skin as well as systemic connective tissue diseases (SCTD), especially lupus erythematosus and vasculitis, including leukocytoclastic vasculitis and Henoch-Schonlein purpura. By DIF, presence of immune complexes in the skin biopsy at various locations, e.g., at the dermoepidermal junction (DEJ), upper dermal blood vessels, cytoid bodies, and intraepidermal intercellular spaces, etc., helps us to arrive at a definite diagnosis. "Lupus band test" (LBT) is most common pattern observed on DIF examination of skin biopsies of patients suffering from SCTDs. Broadly, it is the deposition of immunoglobulins (Igs) at the DEJ in lesional and nonlesional skin with IgM being the most frequent deposit. In addition, DIF microscopy of the skin has also disclosed antibodies bound to epidermal cell nuclei in several connective tissue disorders also known as in vivo ANA (antinuclear antibody) phenomenon or epidermal nuclear staining (ENS) which presents as keratinocyte nuclear fluorescence [Figure - 1]. In 88% of the cases, connective tissue disorders could be predicted by the presence of antibodies against the epidermal cell nuclei of the skin. [1] Circulating ANAs are commonly found in patients with SCTDs.
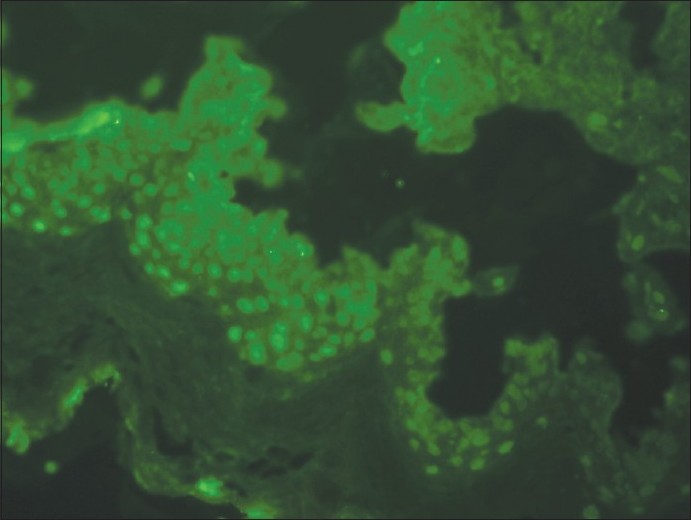 |
| Figure 1: Direct immunofluorescence photomicrograph of skin biopsy showing IgG reactive 2+ diffuse nuclear staining in epidermal cells (ANA in vivo) (×400) |
Immunofluorescence Characteristics
ANA in vivo has been observed in both lesional skin and normal skin. In addition to skin, in vivo ANAs can also be seen in diseased kidney, oral mucosa, and lung tissues in SCTDs. [2] IgG class of antibody is the most common type of Ig found in ANA in vivo; however, less commonly, IgM and IgA can also be found. [3] Four different patterns of ENS (viz. speckled, homogenous, nucleolar, and rim) have been reported in the literature, with speckled pattern being the commonest type. [4] The pattern of in vivo ANA can provide some diagnostic information. The homogeneous in vivo ANA pattern, though seldom found, occurs exclusively in systemic lupus erythematosus (SLE). The nucleolar pattern is very specific for scleroderma. Except for homogeneous pattern, in vivo ANAs do not discriminate better between the various SCTDs than do serum antibodies.
Clinical Correlation
The frequency with which in vivo ANA in skin occurs in various SCTDs varies between 2.6 and 17.8% in different studies. [5],[6] It occurs in 19% of cases with SLE, in 32% of mixed connective tissue disease, in 22% of scleroderma, in 20% of cutaneous vasculitis, in 18% of polymyositis, in 33% of Sjogren′s syndrome, but is absent in cases with rheumatoid arthritis. [7] The diagnostic value of in vivo ANA in differentiating between the various connective tissue disorders is low with the exception of SLE as mentioned above. [1] In SLE patients with in vivo ANA, the incidence of nephropathy is significantly lower (P<0.01), regardless of LBT positivity.
Serological Correlation
Serologically, 98% of patients showing ENS have circulating ANAs by indirect immunofluorescent testing. The patterns of ENS in the skin biopsy specimens correlate with that of serum ANA in the majority of cases. [3],[8] The speckled pattern of ENS is found to be most often associated with serum antibodies to either nuclear ribonucleoprotein (RNP) or Smith (Sm) antigen. [1],[9] These two antigens are constituents of extractable nuclear antigen (ENA). ENA is a saline-soluble (extractable) nuclear antigen with several distinct antigenic sites. One is nuclear RNP that is RNase sensitive and the other is resistant to RNase and is identical to Sm antigen.
It has also been noted that some patients with serum antibodies to ENA did not display in vivo ANA on skin biopsies and vice versa. [4] Some authors have also demonstrated in vivo nuclear staining to be present in one tissue but absent in other tissues of same patients even when biopsies were performed at the same time and processed in the same way. [2] Moreover, no difference was detected between diseased and normal skin for the occurrence of in vivo ANA and also no association has been observed between this phenomenon with immune deposits at DEJ or in subepidermal vessels. [3]
Pathogenesis of Antinuclear Antibody In Vivo
The exact pathogenesis of ANA in vivo remains obscure, but the explanation that it is a simple artifact seems to be quite untenable. Tuffanelli in 1975 proved that the phenomenon is not an artifact, as shown by its repeated observations at various time points in the same patient. [10] Gilliam (1975) and Iwatzuki et al. (1982) maintained that it is an in vitro phenomenon occurring only in relation to high titers of anti-RNP antibody in the blood and attributed ENS to tissue contamination occurring during excision of the skin specimen. [11],[12] Izuno hypothesized that certain permeability-enhancing co-factors, in addition to consistently high titers of RNP antibodies, may be necessary to permit penetration of anti-RNP antibody into the nuclei of living epidermal cells. [13] However, it was later shown that ANA in vivo occurs with Igs other than IgG and not only with low titers of circulating ANA, but even in their absence. [14],[15] The in vivo speckled nuclear staining for Igs within keratinocytes of lesional and nonlesional skin is correlated to antibodies to nuclear RNP and Ro. Relocation of nuclear and cytoplasmic Ro antigens to the cell surface has been implicated as a key event in permission of binding of autoantibodies. Ultraviolet light exposure, viral infection, and estrogen treatment of cultured keratinocytes have been shown to displace Ro antigen. The selective association between in vivo ANA in the skin and non-histone nucleoprotein antibodies in blood suggests it to be a true in vivo phenomenon.
Conclusion
The presence of in vivo ANA in clinically healthy skin is a phenomenon with a high predictive value for SCTDs. [1] However, compared with circulating ANAs, its diagnostic value in discriminating between the various SCTDs is very low. Furthermore, deposition of IgG in epidermal cell nuclei in speckled pattern appears to correlate with high-titres of serum antibody to ENA and is an immunopathologic marker for a subset of SCTDs. These findings re-emphasize the importance of cutaneous immunopathology in the diagnosis and management of patients with SCTDs.
| 1. |
Velthuis PJ, Kater L, van der Tweel I, Meyling FG, Derksen RH, Hene RJ, et al. In vivo antinuclear antibody of the skin. Diagnostic significance and association with selective antinuclear antibodies. Ann Rheum Dis 1990;49:163-7.
[Google Scholar]
|
| 2. |
Williams WV, Barjenbrach P, Adelstein E, Sharp CS, Walker SE. The clinical significance of the in vivo antinuclear antibody phenomenon. Arch Pathol Lab Med 1986;110:798-802.
[Google Scholar]
|
| 3. |
Prystowsky SD, Tuffanelli DL. Speckled (particulate) epidermal nuclear IgG deposition in normal skin. Correlation of clinical features and laboratory findings in 46 patients with a subset of connective tissue disease characterized by antibody to extractable nuclear antigen. Arch Dermatol 1978;114:705-10.
[Google Scholar]
|
| 4. |
Kallenberg CG, de Jong MC, Walstra TM, Kardaun S, The TH. In vivo antinuclear antibodies (ANA) in biopsies of normal skin: Diagnostic significance and relation to serum ANA. J Rheumatol 1983;10:733-40.
[Google Scholar]
|
| 5. |
Burrows NP, Bhogal BS, Russel Jones R, Black MM. Clinicopathological significance of cutaneous epidermal nuclear staining by direct immunofluorescence. J Cutan Pathol 1993;20:159-62.
[Google Scholar]
|
| 6. |
Rao R, Balachandran C. Epidermal nuclear staining: A distinct reaction pattern in connective tissue diseases. Indian J Dermatol Venereol Leprol 2007;73:120-1.
[Google Scholar]
|
| 7. |
Rodrigues CJ, deOliveira RM, Taniwaki NN, Bueno C, Marchiori P, Cossermelli W. Epidermal nuclear immunoglobulin deposition in connective tissue diseases. Rev Hosp Clin Fac Med Sao Paulo 1990;45:154-7.
[Google Scholar]
|
| 8. |
Gilliam JN, Smiley JD, Ziff M. Correlation between serum antibody to extractable nuclear antigen and immunoglobulin localization in epidermal nuclei, abstracted. Clin Res 1974;22:611A.
[Google Scholar]
|
| 9. |
Shu S, Provost T, Croxdale MB, Reichlin M, Beutner EH. Nuclear deposits of immunoglobulins in skin of patients with systemic lupus erythematosus. Clin Exp Immunol 1977;27:238-44.
[Google Scholar]
|
| 10. |
Tuffanelli DL. Cutaneous immunopathology: Recent observations. J Invest Dermatol 1975;65:143-53.
[Google Scholar]
|
| 11. |
Gilliam JN.The significance of cutaneous immunoglobulin deposits in lupus erythematosus and NZB/NZW F 1 hybrid mice. J Invest Dermatol 1975;65:154-61.
[Google Scholar]
|
| 12. |
Iwatsuki K, Tagami H, Imaizumi S, Ginoza M, Yamada M. The speckled epidermal nuclear immunofluorescence of mixed connective tissue disease seems to develop as an in vitro phenomenon. Br J Dermatol 1982;107:653-7.
[Google Scholar]
|
| 13. |
Izuno GT. Observations on the in vivo reaction of antinuclear antibodies with epidermal cells. Br J Dermatol 1978;98:391-8.
[Google Scholar]
|
| 14. |
Parodi A, Nunzi E, Rebora A. The speckled immunofluorescence of epidermal nuclei as an in vivo phenomenon. Br J Dermatol 1985;112:555-8.
[Google Scholar]
|
| 15. |
Sousa JX, Miyamoto D , Zimbres JM, Costa DV, Aoki V. Clinicopathological evaluation of in vivo epidermal nuclear fluorescence. Clin Exp Dermatol 2009;34:314-8.
[Google Scholar]
|
Fulltext Views
3,224
PDF downloads
3,307





