Translate this page into:
Incidence of leprosy in Firozabad district (Uttar Pradesh)
2 Department of Clinical Medicine, National Jalma Institute for Leprosy and Other Mycobacterial Diseases, Taj Ganj, Agra, India
Correspondence Address:
Anil Kumar
National Jalma Institute for Leprosy and Other Mycobacterial Diseases, Taj Ganj, Agra - 282 004
India
| How to cite this article: Kumar A, Girdhar A, Chakma JK. Incidence of leprosy in Firozabad district (Uttar Pradesh). Indian J Dermatol Venereol Leprol 2018;84:403-407 |
Abstract
Objective: To assess incidence of leprosy in Firozabad District (U.P.).
Materials and Methods: A random sample of 148,061 population was covered by this second survey, spread over 259 units (230 rural/29 urban). The survey was conducted between March 2011 and November 2012. Clinically confirmed cases detected in known disease-free population were labeled as incident cases and treated.
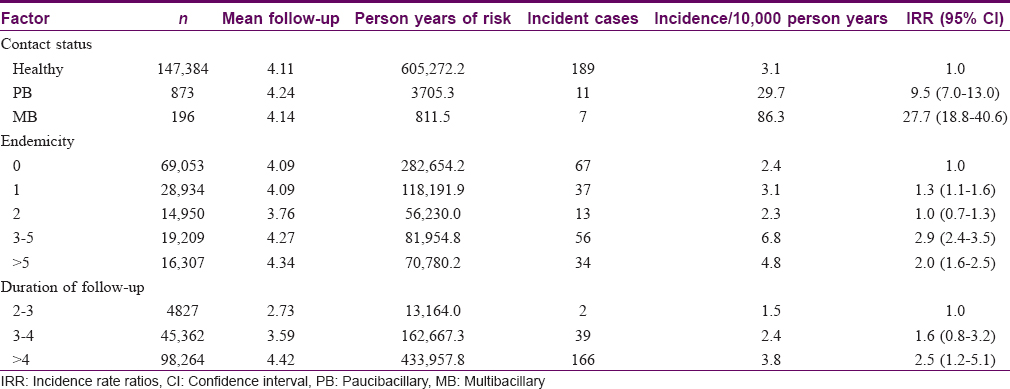
Results: The overall incidence rate of leprosy was found to be 3.4 per 10,000 person years; In healthy contacts it was 3.1, in paucibacillary contacts 29.7 while it was 89.3 in multibacillary contacts. The differences in incidence rate of these three groups were significant (P < 0.001). Incidence rate was significantly higher by age; 1.1 in persons <15 years to 8.0 in those >44 years of age, and in high endemicity areas with three or more cases. In terms of incidence rate ratio (95% confidence interval), the incidence for ages 15–24 years was 3.2 times significantly higher than for those under 15 years, 5.3 times (4.3–6.5) in ages 30–44 years and 7.0 times (5.6–8.7) for age ≥45 years. Incidence rate ratio was also significantly higher in paucibacillary contacts, by 9.5 times (7.0–13.0) and 27.7 times (18.8–40.6) in multibacillary contacts, as compared to healthy controls. Incidence rate ratio (95% confidence interval) was significantly higher by 2.9 times (2.4–3.5) in areas with endemicity status of 3 to 5 cases and by 2.0 times (1.6–2.5) in areas with >5 cases as compared to areas with no endemicity. It was 2.4 times more (1.6–3.5) in Narkhi, 2.4 times higher (1.7–3.5) in Tundla and 3.0 times higher (2.1–4.5) in Aravon blocks than in Aeka block of the district. Incidence rate was also found to be significantly higher (3.7) among females, 1.3 times higher (1.1–1.5) than in males (2.9). Incidence rate ratio (95% confidence interval) was also 2.5 times higher (1.2–5.1) among persons having reported disease of greater than 4 years in comparison to 1.5 in persons having disease for 2 to 3 years.
Limitations of Study: None to the best of our knowledge.
Conclusion: The present study suggests that incidence rate of leprosy is significantly higher among persons of above 15 years, in females, among contacts of paucibacillary/multibacillary disease, in areas where >3 leprosy cases were found and in Tundla, Narkhi and Aravon blocks in Firozabad district.
Introduction
Epidemiological evaluation of leprosy is an important public health tool to monitor its progress on account of new transmission taking place in an area. This also helps to understand the trend of disease and causes of transmission following public health intervention and its impact. Although the leprosy control program reports a significant decline in the leprosy case load all over the country based on trends in prevalence and new case detection, the incidence of leprosy is never reported. It has been mentioned that measuring incidence of leprosy is not easy.[1] The incidence of leprosy suggests the levels of ongoing transmission in various groups and subgroups.[2]
Leprosy control strategies had been based on reported new leprosy cases in total population of an area assuming high community coverage for detection and treatment. However, for some practical reasons this has not happened in India. Therefore, this variable coverage in different areas of the country has given variable results. The drawback with basing control strategies on reported new leprosy cases is that there remains a significant number of hidden or unreached leprosy cases that are responsible for ongoing transmission in an otherwise healthy population. The measurement of incidence of disease is thus important for planning to interrupt ongoing transmission, if any, in the community.
Several attempts have been made to estimate the incidence of leprosy in Tamil Nadu,[2],[3],[4],[5],[6] Uttar Pradesh,[7],[8] Maharashtra,[9],[10] and elsewhere [11] Studies from South India have shown that household familial contacts of index leprosy cases had higher risk of developing leprosy than in nonfamilial contacts.[3],[4] Although several studies from Uttar Pradesh,[7] Mumbai,[9] and Wardha [10] had looked into crude incidence, a study from Agra in Uttar Pradesh [8] had presented detailed data on observed incidence. The present study was conducted in Firozabad District (Uttar Pradesh) between March 2011 and November 2012 in a population previously surveyed between 2006–2009, in order to determine observed incidence.
Materials and Methods
Background of the study
The Firozabad district was reporting a very low number of leprosy cases. In 2005–2006, the reported prevalence of leprosy was 0.17 per 10,000 population. A survey was undertaken in the district starting from October 2006 to March 2009, and the new case detection rate was found to be 7.57 per 10,000 population [12] in 9.83 lakh population.
Sample size
Using new case detection rate of 7.57 per 10,000 populations, error rate as 0.20, a sample size of 129,099 was estimated. Assuming coverage 50% population on account of migration and other factors, inflated sample size was 258,198. This sample was randomly chosen from 2006 to 2009 surveyed population and finally 148,453 could be resurveyed for estimating the incidence of leprosy.
Defining incidence of leprosy
A new, active, confirmed, untreated leprosy case detected among an earlier surveyed population (2006–2009) known to be leprosy free is defined as an incident case. A whole body examination of all persons in the targeted population was conducted. The mean duration between two surveys was 4.1 years. Both surveys were conducted using cross-sectional study design to interview/examine only once at the time of visit.
Incidence rate
This was calculated using the formula:
New leprosy cases detected as incident cases divided by (earlier disease-free population × duration in years between two surveys) and multiplied by 10,000 = Incidence rate per 10,000 person years.
Defining contact status
A contact is a person living with an index leprosy case in the family. Therefore, healthy contact is a person with no known leprosy case in the family, paucibacillary contact with at least one paucibacillary index case and multibacillary contact with at least one multibacillary index case with/without other cases in the family.
Defining endemicity
There is no set definition of endemicity. The endemicity of leprosy is therefore defined based on leprosy cases per village or urban unit such as 0, 1, 2, 3, 4, 5 and >5 in this study.
Data analysis
The data was computerized and analyzed using Statistical Package for the Social Sciences (SPSS v. 18). Incidence rate of leprosy is calculated as number of incidence cases per 10,000 person years of follow-up. Kaplan–Meier survival method was used to estimate incidence with time and log-rank test was used to compare the hazard probabilities.[13] To compare within the groups such as age, sex, rural–urban, etc., incidence rate ratio and 95% confidence interval of incidence rate ratio were calculated to assess the significance.[14]
Results
The overall incidence rate of leprosy in the district was observed to be 3.4 per 10,000 person years of observations [Table - 1]. The observed incidence by age suggests that incidence of leprosy increased from 1.1 per 10,000 person years in young age (<15) to 3.7 in adult ages (15–29) and further to 6.0 by ages 30 to 44 and again to 8.0 beyond 44 years of age. The incidence rate suggests significant differences in incidence rate ratio from age <15 to 15–29 [incidence rate ratio (95% confidence interval) =3.2 (2.6–4.1)], to age group 30–44, 5.3 (95% confidence interval = 4.3–6.5) and for age group above 44, 7.0 (95% confidence interval = 5.6–8.7).
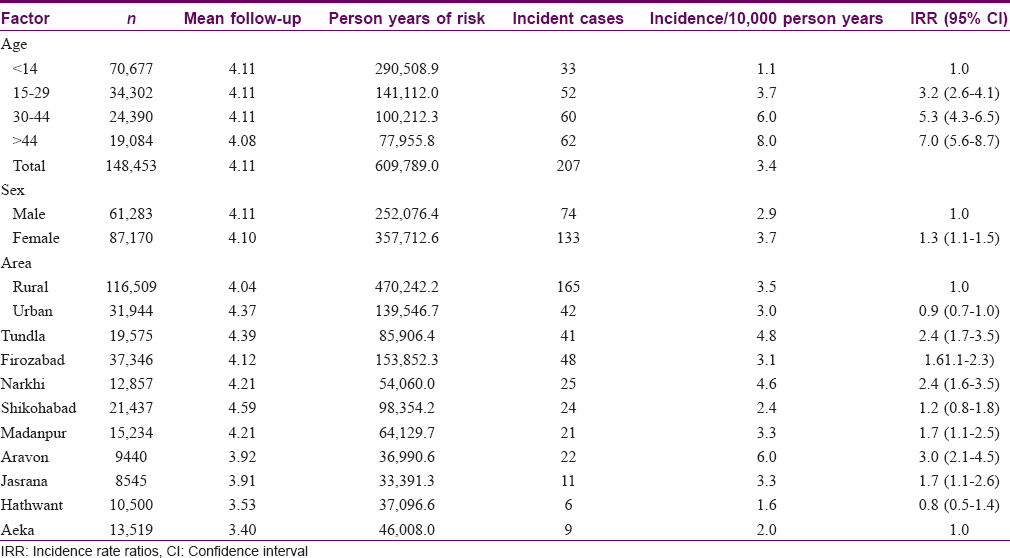
The clinical presentation of incident cases suggests that detection was not delayed, as 189 (91.3%) cases detected were of paucibacillary type, and 142 (68.6%) of these were with single skin lesion only. Another 47 (22.7%) presented with 2–5 skin lesions and the remaining 18 (8.7%) were detected with multibacillary disease. Of the 207 incident cases, 33 cases (15.9%) were children (under 15).
The observed incidence rate was 2.9 in males while it was 3.7 in females (95% confidence interval = 1.1–1.5), which was significantly higher. Incidence rate of leprosy was 3.5 in rural areas and 3.0 in urban areas (95% confidence interval = 0.7–1.0), which was not statistically significant. Of the 9 blocks, incidence rate was found to be lowest (below 2) in Hatwant (1.6) followed by Aeka (2.0) and Sikohabad (2.4) while higher incidence (above 4) was found in Narkhi (4.6) and Tundla (4.8) and the highest rate of 6.0 was found in in Aravon block of the district [Table - 1]. Assuming the incidence rate in Aeka block as base value, it is observed that the incidence rate of leprosy was 3.0 times (95% confidence interval = 2.1–4.5) in Aravon, 2.4 times (95% confidence interval = 1.7–3.5) in Tundla, 2.4 times (95% confidence interval = 1.6–3.5) in Narkhi and so on. However, incidence rate in Shikohabad was 1.2 times that of Aeka (95% confidence interval = 0.8–1.8) and 0.8 times lower in Hathwant (95% confidence interval = 0.5–2.6); both are not significant.
The incidence rate in healthy controls was 3.1 in households (where there was no index case of leprosy earlier). The incidence rate was (29.7) significantly high, as much as 9.5 times (95% confidence interval = 7.0–13.0), and also higher (89.3), and 27.7 times (95% confidence interval = 18.8–40.6), among multibacillary contacts in comparison to healthy controls.
The incidence rate was observed to be 2.4 in areas with leprosy endemicity of zero (or <1). The incidence rate was 1.3 times (95% confidence interval = 1.1–1.6) significantly higher in areas with endemicity level of 1, but not different in areas with endemicity level of 2 (95% confidence interval = 0.7–1.3). However, incidence levels were significantly higher in areas with endemicity levels of 3–5 and >5 cases in comparison to incidence rate in areas with zero endemicity. In areas with endemicity levels of 3 to 5, incidence was 6.8 per 10,000 person years, 2.9 times (95% confidence interval = 2.4-3.5) in comparison to areas with endemicity zero, and 4.8 in areas with endemicity of >5 [incidence rate ratio (95% confidence interval) = 2.0 times (1.6–2.5) of the base value]. It was also observed that as the duration of follow-up increased, the incidence of disease was higher. No incident case of leprosy was noted before 2 years of follow-up, but incidence rate of 1.5 per 10,000 person years was noted in areas resurveyed after 2–3 years, 2.4 during 3–4 years of follow-up and 3.8 beyond 4 years of follow-up. The incidence rate ratio was significantly higher i.e., 2.5 times (95% confidence interval = 1.2–5.1) among persons having disease for over 4 years.
Discussion and Conclusion
The incidence rate of leprosy among various population subgroups gives an understanding about transmission pattern of disease and also is an indicator of the impact of ongoing public health interventions and effectiveness.[2] The overall incidence of leprosy in the study areas of Firozabad district is 3.4 and 3.1 in healthy controls. It was significantly increased by 9.5 times (95% confidence interval = 7.0–13.0) among paucibacillary contacts and 27.6 times (95% confidence interval = 18.8–40.6) among multibacillary contacts (log-rank test = 265, degrees of freedom = 2, P < 0.001). The incidence among multibacillary contacts has been highest and about three times as that in paucibacillary contacts. An earlier study done in Agra district [8] also found almost similar results, with an incidence rate of 4.6 in healthy controls, increased to 41 (8.9 times) in paucibacillary contacts and further to 131.3 (28.5 times) in multibacillary contacts. This study also suggests that multibacillary contact had three times higher infectivity than that of paucibacillary contacts [Figure - 1]. This probably confirms that exposure to large quantum of infection in familial contacts of multibacillary index cases gives rise to genetic predisposition among contacts, and thus receptivity of infection increases. The behavioral practice of defecation in the open, as is widely prevalent both in rural and urban slums areas, increases exposure to infection, and thus the development of disease. The observed incidence rate varied from 1.6 in Hatwant block to 6.0 in Aravon block. Incidence rate ratio suggests that a much higher incidence from base value of two or more times is found in Narkhi, Tundla and Aravon blocks than in Aeka block, which is significant. The observed high endemicity (proxy to force of infection) to help in transmission and high significant increase in incidence rate have been observed in areas with high endemicity of ≥3 leprosy cases than in areas with lower endemicity status <3. This is completely a new finding to the best of authors' knowledge.
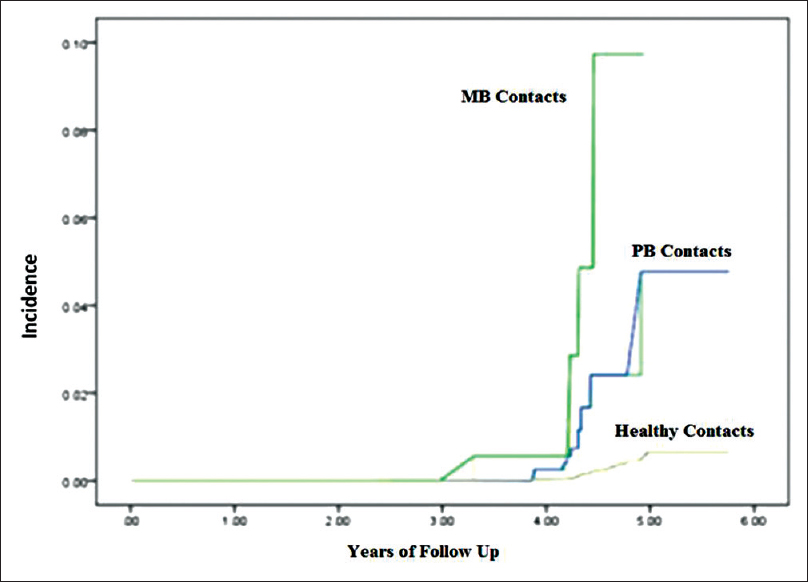 |
| Figure 1: Incidence of leprosy in Firozabad District |
The incidence of disease is found to be high in higher ages and this may be due to increasing length of exposure and incubation period of disease. This corroborates with increasing risk of incidence with longer follow-up time [Table - 1] and [Table - 2].
Similar to other studies,[3],[11] the present study indicates high incidence rate (3.7) in females, which is 1.3 times (95% confidence interval = 1.1–1.5) that of 2.9 in males and this is different from the observations in Agra.[8] One of the reasons could be more thorough (total body) examination undertaken.
Time interval between two surveys, in determining incidence rates, has been a subject of debate, there being no unanimity for length of follow-up. In this study, the average interval between the two surveys was 4.11 years. The findings of this study are based on definite cases of leprosy that had developed into defined clinical types of the disease. It is possible that some of the early cases that might have appeared in the intervening period may have self-healed. Had the resurvey been done early (6–12 months interval), those self-healed cases too could have been picked up to further add to the incidence. However, the number of such cases is expected to be very few.
To conclude, new leprosy cases are continuing to appear and the incidence rate is still much higher (3.4 per 10,000 person years) compared to reported new case detection rate of 0·587/10,000 (proxy for incidence) by district health authorities (unpublished, 2009). Larger numbers of incident cases were observed among the nonfamilial healthy controls of leprosy but a 10–29 fold higher incidence rate was observed in familial contacts of index cases. The reason of such a situation could be related to daily behavior of index leprosy cases of defecation within the villages in open areas, which provide aerosol effect for the infection to transmit and also increase the genetic predisposition. A similar or more alarming situation may exist in some urban slums where localized transmission is ongoing.
Future research, using large numbers of contacts who could be resurveyed, can throw more light on the transmission patterns with reference to contact's status, and corroborating findings of infection in the soil. Special observations on the treatment status of contacts and the duration of untreated disease can also lead to understanding as to how much multidrug therapy has really helped in containing transmission, or if it could decline naturally as a function of improved hygiene and sanitation practices.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given their consent for images and other clinical information to be reported in the journal. The patients understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Limitation of study
None to best of authors' knowledge.
Financial support and sponsorship
ICMR grant ID No. 2009-5180.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Declercq E. Reflections on the new WHO leprosy indicator: The rate of new cases with grade 2 disabilities per 100,000 population per year. Lepr Rev 2011;82:3-5.
[Google Scholar]
|
| 2. |
Rao PS, Karat AB, Kaliaperumal VG, Karat S. Transmission of leprosy within households. Int J Lepr Other Mycobact Dis 1975;43:45-54.
[Google Scholar]
|
| 3. |
Jesudasan K, Bradley D, Smith PG, Christian M. Incidence rates of leprosy among household contacts of “primary cases”. Indian J Lepr 1984;56:600-14.
[Google Scholar]
|
| 4. |
Jesudasan K, Bradley D, Smith PG, Christian M. Time trends in the analysis of incidence rate of leprosy among household contacts. Indian J Lepr 1984;56:792-806.
[Google Scholar]
|
| 5. |
Sundar Rao PS, Jesudasan K, Mani K, Christian M. Impact of MDT on incidence rates of leprosy among household contacts. Part 1. Baseline data. Int J Lepr Other Mycobact Dis 1989;57:647-51.
[Google Scholar]
|
| 6. |
George R, Rao PS, Mathai R, Jacob M. Intrafamilial transmission of leprosy in Vellore town, India. Int J Lepr Other Mycobact Dis 1993;61:550-5.
[Google Scholar]
|
| 7. |
Sharma VK. The epidemiologic significance of leprosy within the household. Int J Lepr Other Mycobact Dis 1968;36:1-16.
[Google Scholar]
|
| 8. |
Kumar A, Girdhar A, Girdhar BK. Incidence of leprosy in Agra district. Lepr Rev 2007;78:131-6.
[Google Scholar]
|
| 9. |
Ganapati R, Revankar CR. Associated cases in the families of school children with leprosy. Lepr Rev 1978;49:43-6.
[Google Scholar]
|
| 10. |
Ranade MG, Joshi GY. Long-term follow-up of families in an endemic area. Indian J Lepr 1995;67:411-25.
[Google Scholar]
|
| 11. |
Pönnighaus JM, Fine PE, Sterne JA, Bliss L, Wilson RJ, Malema SS, et al. Incidence rates of leprosy in Karonga district, Northern Malawi: Patterns by age, sex, BCG status and classification. Int J Lepr Other Mycobact Dis 1994;62:10-23.
[Google Scholar]
|
| 12. |
Kumar A, Girdhar A, Chakma JK, Girdhar BK. Detection of previously undetected leprosy cases in Firozabad District (U.P.), India during 2006-2009: A short communication. Lepr Rev 2013;84:124-7.
[Google Scholar]
|
| 13. |
Collett D. Modelling Survival Data in Medical Research. USA: Chapman & Hall; 1996. p. 40-3.
[Google Scholar]
|
| 14. |
Sahai H, Khurshid A. Statistics in Epidemiology: Methods, Techniques and Applications. London: CRC Press; 1995. p. 184-92.
[Google Scholar]
|
Fulltext Views
3,873
PDF downloads
1,815





